Abstract
BACKGROUND AND PURPOSE: Reported CT angiographic (CTA) subtraction methods are not simple, robust, or real time. We investigated a novel technique for semiautomated digital subtraction CTA of the intracranial and extracranial arteries.
METHODS: Thirty patients underwent precontrast (low milliampere-seconds) and postcontrast (pitch, 1.5; collimation, 1–2.5 mm) helical imaging with a vacuum-type head holder to facilitate image registration and minimize movement. A reconstructed three-dimensional model of the precontrast bone dataset was subtracted from the postcontrast dataset to produce subtracted maximum-intensity-projection angiograms. Experienced (operator 1) and less-experienced (operator 2) staff performed the standard and subtraction reconstructions, and image generation time and quality (graded 1–5) were compared. A third operator blinded to the method assessed the hard-copy image quality.
RESULTS: Image quality with subtraction postprocessing was significantly better with both operators (operator 1, mean improvement of 0.87 grade, median improvement of 1 grade, P < .001; operator 2, mean improvement of 0.63 grade, median improvement of 1 grade, P < .001). Hard-copy image quality was better with the subtraction method (operator 1, P > .001; operator 2, P < .001). Blood vessels at the base of the brain were better demonstrated on subtraction images in 13 of 14 examinations. For the less experienced operator, the reconstruction time was significantly less with the subtraction method than with the conventional method (mean, 7.5 vs 10.1 minutes; P = .001).
CONCLUSION: When separation of the vasculature from bone is important and technically difficult, digital subtraction CTA offers a potential advantage. This semiautomated technique is fast and easy to learn, and variably experienced staff can use it.
Helical CT angiography (CTA) is a useful, noninvasive imaging technique for the evaluation of both the intra- and extracranial vasculature (1–4). It has the advantages of multiplanar image reconstruction, ready availability, low cost, and speed of acquisition, with reduced motion artifacts. However, separating vessels from bone or perivascular calcification can be difficult, particularly in areas such as the skull base. This limitation can be a barrier to its use in clinical practice (5). A further difficulty is that the reconstruction of spiral datasets by using conventional methods is time-consuming and operator dependent. A subtraction technique could offer the potential for an effective, rapid, and semiautomated (ie, operator-independent) procedure with improved distinction between bones and vessels.
A few methods of subtraction have been reported with CTA, but none has yet provided a simple, robust, real-time method acceptable to patients. The techniques described so far require specially adapted equipment or computer subtraction algorithms (6, 7). Our purpose was to investigate the usefulness of a novel, rapid technique of digital subtraction CTA (DSCTA) applied to the intracranial and extracranial arterial vasculature to determine if it overcomes these drawbacks. We present our initial findings here.
Methods
Our technique of subtraction CTA is simple, practical, and rapid, and it is performed on the workstation provided with the CT scanner (Elscint CT Twin; Philips, UK). A low-dose (33-mAs) helical acquisition was performed by using a pitch of 1.5, a beam collimation of 1–2.5 mm (1 mm for intracranial vasculature and 2.5 mm for extracranial vasculature). and a reconstruction interval equivalent to 50% of the beam collimation. Immediately after the first acquisition, the table is repositioned precisely at the original start position, and the injection of contrast material commenced. Typically, 100 mL of Omnipaque 240 iohexol was administered at a rate of 3 mL/s via a 20-gauge intravenous cannula in a cubital vein. The postcontrast helical acquisition was performed 18 seconds after the start of the injection by using exactly the same factors as before, but with the maximum milliamperage-seconds allowed; typically 159 mAs.
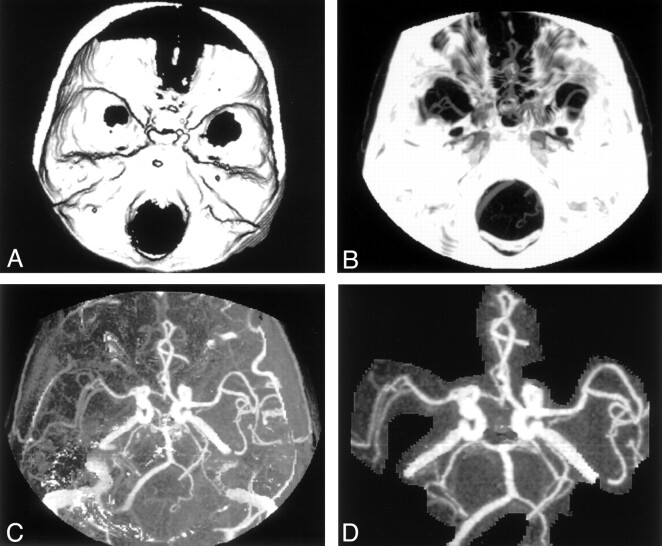
The scanner reconstructs the images of the pre- and postcontrast datasets by using identical an zoom factor and spatial coordinates (X, Y, and Z). These images are then transferred to an offline workstation, a Silicon Graphics O2 Omnipro (Mountainview, CA). Initially, processing of two datasets in the same application was not permitted by the workstation. This problem was resolved with a minor modification of the software, which then permitted us to edit the study number on the header files of each section. A three-dimensional model of the bones and any other calcification was reconstructed from the first dataset (Fig 1A). The bone model is then combined with the postcontrast dataset, and a maximum intensity projection (MIP) is created. From this, the bone model is then automatically subtracted to produce the CTA MIP with the bone removed (Fig 1B and C). The final images can be produced with little or minimal editing (Fig 1D). By setting the bone opacity to 0 or by giving the bone a unique color, volume-rendered or surface-shaded images could also be easily reconstructed from the contrast-enhanced dataset.
Fig 1.
Image processing for subtraction CTA.
A, Three-dimensional model of the skull is created from precontrast dataset.
B, MIP created from the contrast-enhanced dataset.
C, Subtracted image after removing A from B, before any editing.
D, Subtracted image with minimal editing.
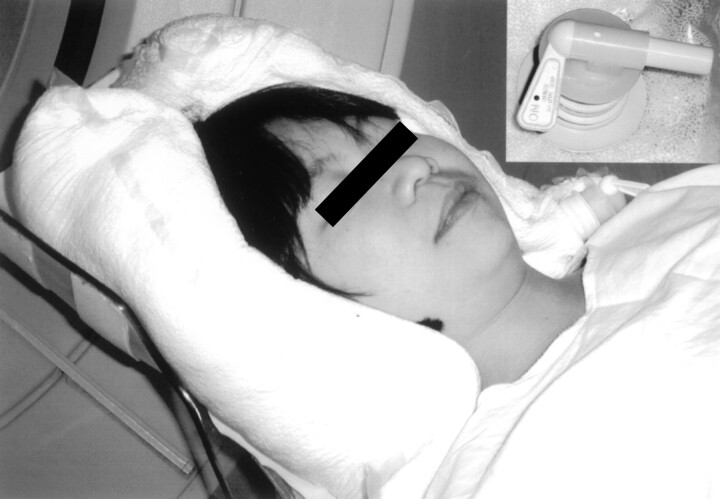
To minimize patient movement between the pre- and postcontrast acquisitions and to facilitate precise registration of images, we developed a vacuum type of head holder. This consisted of a 100-μm-thick polyethylene casting bag filled with polystyrene beads and a suction port. This material is pliable yet extremely resistant to tears. The bag is firmly molded around the posterior aspect of the skull, the sides of the head, and the angle of the jaw when conventional hospital suction is applied, thus minimizing patient movement (Fig 2). This motion restriction device can be simply and inexpensively made by using readily available materials. The incorporation of the suction port into the bag may require some technical skill. In our case, this was easily accomplished by the CT engineer (P.C.). The total time for the examination was typically less than 15 minutes, with the actual CT scanning taking less than 90 seconds. A detailed explanation of the procedure was given to the patient, with an emphasis on the need for him or her to keep absolutely still throughout the whole scan. The patients’ tolerance for the procedure and the head restraint was generally good, and no technical failures occurred.
Fig 2.
The restraining device is molded around the back of the patient’s head and the angles of the jaw. Inset shows the valve for the suction connection
To evaluate the practical usefulness of this technique, it was performed in 30 patients. The selection of patients was done nonconsecutively over a period of 6 months, and a balance was kept between patients being examined for known or suspected intracranial aneurysms (14 of 30) and those with carotid occlusive disease (10 or 30) (Table 1). Two operators reconstructed the MIP angiograms the Omnipro workstation for the 30 cases in two ways: 1) by using the standard technique of thresholding and manual extraction of bone with the postcontrast dataset only and 2) by using the subtraction CTA method described above. One of the operators (operator 1 [V.K.J.], a trainee neuroradiologist) was experienced in the postprocessing of standard CTAs and had performed 20 such reconstructions before the study. The other (operator 2 [D.A.], a neuroradiographer) had minimal training and experience in reconstructing CTAs and had done performed only five such procedures before. The time from the loading of the axial volume data to the generation of acceptable images in three orthogonal planes (axial, coronal, and sagittal) with each method was recorded.
TABLE 1:
Patient selection
| Indications for CTA and DSCTA | No. of Patients (n = 30) |
|---|---|
| Suspected or known intracranial aneurysm | 14 |
| Carotid occlusive disease | 10 |
| Skull-base tumors | 3 |
| Cerebral venous thrombosis | 3 |
The quality of image produced by each method was carefully assessed and given a grade of 1–5 (Table 2). An independent third operator (operator 3 [E.M.T.], an experienced consultant neuroradiologist) evaluated the hard-copy images produced by the two operators by using the same scale. Operator 3 was blinded to the method of image reconstruction. In the examinations performed to detect intracranial aneurysms, the third operator separately recorded the image quality for the depiction of vessels in the base of skull and in the circle of Willis.
TABLE 2:
Grading system used for the assessment of image quality
| Grade and Definition | Intracranial Findings |
Neck Findings |
||
|---|---|---|---|---|
| Circle of Willis | Skull-Base Vessels | Carotids | Vertebral Arteries | |
| 1, nondiagnostic | Obscured | Obscured | Obscured | Obscured |
| 2, poor quality | Poorly visualized | Obscured | Poorly visualized | Obscured |
| 3, acceptable | Clearly seen | Obscured | Clearly seen | Obscured |
| 4, good quality | Clearly seen | Partly obscured | Clearly seen | Partly obscured |
| 5, excellent | Clearly seen | Clearly seen | Clearly seen | Clearly seen |
Standard statistical tests for symmetry and significance were applied to the observations of image quality and time for reconstruction. Unweighted κ statistics with 95% confidence intervals (CI) were used to assess the level of observer agreement on image quality achieved by the two methods. (A κ value of <0.20 implies poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; and 0.81–1.0, excellent agreement).
Results
The image quality achieved with DSCTA postprocessing on the workstation was found significantly better than that of conventional CTA, for both operator 1 and operator 2 (P = .003 and P = .014, respectively).
Using DSCTA, operator 1 achieved a mean improvement in image quality over CTA of 0.87 grade and a median improvement of 1 grade, whereas operator 2 achieved a mean improvement of 0.63 grade and a median improvement of 1grade (Table 3).
TABLE 3:
Summary of quality scores for images produced with CTA and DSCTA
| Test | Operator 1 (n = 30) |
Operator 2 (n = 30) |
||||
|---|---|---|---|---|---|---|
| Mean | Median | SD | Mean | Median | SD | |
| CTA | 2.50 | 3 | 0.86 | 2.67 | 3 | 0.80 |
| Hard-copy check by operator 3 | 3.07 | 3 | 1.17 | 2.83 | 3 | 1.09 |
| DSCTA | 3.37 | 3 | 0.81 | 3.30 | 3 | 0.95 |
| Hard copy check by operator 3 | 3.97 | 4 | 0.85 | 3.83 | 4 | 0.95 |
| DSCTA minus CTA* | 0.87 | 1 | 0.73 | 0.63 | 1 | 0.56 |
| Hard-copy check by Observer 3† | 0.90 | 1 | 1.03 | 1.00 | 1 | 1.11 |
95% CIs were 0.59, 1.14 for operator 1 and 0.43, 0.84 for operator 2. P values with the t test were <.001 for both operators.
95% CIs were 0.52, 1.25 for operator 1 and 0.58, 1.42 for operator 2. P values with the t test were <.001 for both operators.
A fair level of agreement was obtained between operators 1 and 2 for image quality with CTA (κ = 0.28; 95% CI: 0.008, 0.558) and with DSCTA (κ = 0.29; 95% CI: 0.100, 0.487).
On hard-copy images, the quality of subtraction images produced by both operator 1 and operator 2 was significantly better than that of with conventional CTA (P = .023 for operator 1 and 0.050 for operator 2). The mean and median improvement in image quality noted by the third operator is given in Table 3. Good levels of agreement for image quality were achieved with CTA and DSCTA between operators 3 and 1 (κ = 0.65; 95% CI: 0.50, 0.82 for CTA and κ = 0.66; 95% CI: 0.54, 0.78 for DSCTA). Moderate levels of agreement were noted between operators 3 and 2 (κ = 0.44; 95% CI: 0.28, 0.60 for CTA and κ = 0.46; 95% CI: 0.30, 0.62 for DSCTA).
Operator 3 recorded the image quality separately for the base of the brain and for the circle of Willis in the 14 patients with suspected or known intracranial aneurysms. In 13 of 14 examinations, considerable improvement in image quality of vessels at the base of the brain was achieved with DSCTA, whereas the image quality for the circle of Willis was practically unchanged between the two techniques. With operator 1, the quality of images of the base of the brain in six patients improved from being nondiagnostic or poor with CTA to acceptable or good with DSCTA. The image quality in four patients improved from acceptable to good; in three patients, it improved from good to excellent; and in one, it was equally good with both methods. With operator 2, the quality of the images at the base in nine patients improved from being nondiagnostic or poor with CTA to acceptable or good with DSCTA. The image quality in three patients improved from acceptable to good or excellent, and it was equally good with both methods in two.
In seven of 10 patients who underwent an evaluation of carotid stenosis in the neck, images of the vertebral arteries of sufficient quality were produced by using DSCTA. With manual editing in CTA, separation of the vertebral arteries from surrounding bone proved impossible in all 10 patients.
With the less experienced operator, the time to perform the reconstructions was significantly less with the subtraction method than with the conventional method (mean time, 7.5 vs 10.1 minutes; P = .001). For the experienced operator, the difference in time between the two methods was not significant. The mean times taken by the two operators to perform image reconstruction were 9.2 minutes with CTA and 8.2 minutes with by DSCTA (Table 4).
TABLE 4:
Comparison of the time to assess the images produced with CTA and DSCTA
| Observer (n = 30) | CTA |
DSCTA |
CTA vs DSCTA |
||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | 95% CI | P Value | |
| 1 | 8.44 | 2.47 | 8.88 | 2.89 | −0.44 | −1.19, 0.32 | .249 |
| 2 | 10.07 | 4.55 | 7.53 | 2.85 | 2.53 | 1.08, 3.99 | .001 |
| 1 and 2 (mean) | 9.26 | 3.10 | 8.21 | 2.25 | 1.05 | 0.34, 1.75 | .005 |
Discussion
CTA has an established role as an important tool for noninvasive vascular imaging. The technique is increasingly used in the intra- and extra cranial vasculature, particularly in the detection and evaluation of intracranial aneurysms and vascular occlusive disease in the neck (1–4). Conventional CTA methods depend on postprocessing of the helical data followed by manual editing to remove the bony structures. This approach has significant limitations, especially in the region of the skull base where separation of blood vessels from bone could be difficult, and a low sensitivity for the test could result (5). Manual editing can be time-consuming and less experienced operators may have difficulty in selecting the correct areas to be removed.
Results of our study show that, by using a simple digital subtraction technique to remove bone, the quality of images generated with CTA can be significantly improved. The crucial factors in obtaining good-quality subtraction are 1) avoiding movement artifacts during the entire time of image acquisition and 2) accurate registration of pre- and postcontrast datasets. For their subtraction CTA technique, Gorzer et al (6) used either a stereotactic frame or another atraumatic fixation device to reduce motion artifacts. We found that, to prevent nodding movements, the angles of the jaw had to be included in the restraint. The restriction device that we designed works simply by hardening around the back and sides of the patient’s head when suction is applied. It is nontraumatic and simple to create, and it does not require any modification to any existing head support.
Only a minor addition to the existing image processing software was needed to allow us to combine the two acquisitions. This step is simple compared with those of previously described methods for image registration before subtraction; an example is controlled-orbit helical scanning (7). Gorzer et al (6) used a special subtraction algorithm with an average postprocessing time of 15 minutes. The software patch used in the present study produced images faster, with the average postprocessing time of 8.2 minutes.
For operators with limited experience in image processing, DSCTA offers a potential advantage because of the minimal manual editing required compared with that needed with standard CTA. Thereby, DSCTA may save time, improve quality, and reduce the likelihood of the accidental removal of important data. With experienced operators, time may not be saved, but the image quality is improved. This technique can be easily adapted for other workstations that use different commercially available software.
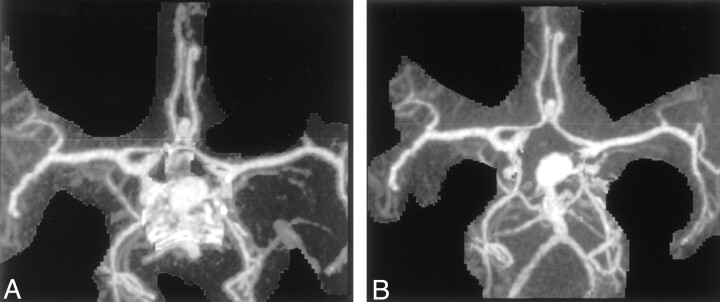
The operators in our study noted one that the one main advantage of DSCTA over standard CTA was the clearer depiction of blood vessels with CTA as they pass through the base of the skull (Figs 3, 4) As expected, delineation of the vessels of the circle of Willis was not significantly improved with the subtraction method, although the MIP display was achieved more rapidly with DSCTA than with CTA. In their study of 36 patients, Imakita et al (7) found that subtraction CTA was superior to CTA in the detection of aneurysms adjacent to bone and found that subtraction CTA was superior or equivalent to conventional angiography in all cases.
Fig 3.
MIP images.
A, MIP CTA image after editing shows residual bone at the base of the skull.
B, MIP DSCTA image better shows the basilar artery, its branches. and the aneurysm.
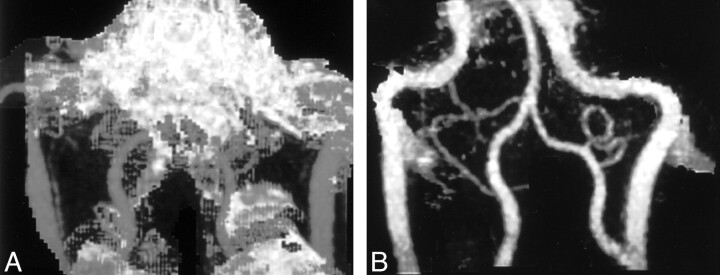
Fig 4.
MIP images.
A, MIP CTA image shows that bone obscures the detail of the distal vertebral arteries.
B, MIP DSCTA image clearly shows clear the vertebral arteries and the posterior inferior cerebellar arteries.
When used for evaluating carotid artery disease in the neck, subtraction CTA offers an additional advantage in that the vertebral arteries can also be assessed on the processed images, whereas they are often lost during manual editing of conventional CTA images.
Because low milliamperage is used for creating the bone mask, this technique produces only a small increase in the radiation dose to the patient. Using our protocol, the effective dose with standard CTA is 0.57 mSv, and this increases to 0.69 mSv with subtraction CTA.
Conclusion
Subtraction CTA offers a significant improvement in image quality and saves time in the noninvasive evaluation of intra- and extracranial vascular disease. These benefits can be achieved with a simple head-restraining device and a minor modification to the image processing software.
Footnotes
Presented at the 39th annual meeting of the American Society of Neuroradiologists, April 2001, Boston, MA.
References
- 1.Ogawa T, Okudere T, Noguchi K, et al. Cerebral aneurysms: evaluation with three-dimensional CT angiography. AJNR Am J Neuroradiol 1996;17:447–454 [PMC free article] [PubMed] [Google Scholar]
- 2.Young N, Dorsch NW, Kingston RJ, et al. Spiral CT scanning in the evaluation of the Circle of Willis. Surg Neurol 1998;50:50–60 [DOI] [PubMed] [Google Scholar]
- 3.White PM, Wardlaw JM, Easton V. Can non-invasive imaging tests accurately detects intracranial aneurysms? A systematic review. Radiology 2000;217:361–370 [DOI] [PubMed] [Google Scholar]
- 4.Sameshima T, Futami S, Morita Y, et al. Clinical usefulness and problems with three dimensional CT angiography for the evaluation of atherosclerotic stenosis of the carotid artery: comparison with conventional angiography, MRA and ultrasound sonography. Surg Neurol 1999;51:300–309 [DOI] [PubMed] [Google Scholar]
- 5.Schwartz RB, Tice HM, Hooten SM, et al. Evaluation of cerebral aneurysms with helical CT: correlation with conventional angiography and MR angiography. Radiology 1994;192:717–722 [DOI] [PubMed] [Google Scholar]
- 6.Gorzer H, Heimberger K, Schindler E. Spiral CT angiography with digital subtraction of extra and intra cranial vessels. J Comput Assist Tomogr 1994;18:839–841 [DOI] [PubMed] [Google Scholar]
- 7.Imakita S, Onishi Y, Hashimoto T, et al. Subtraction CT angiography with controlled orbit helical scanning for detection of intracranial aneurysms. AJNR Am J Neuroradiol 1998;19:291–295 [PMC free article] [PubMed] [Google Scholar]