Abstract
Summary: During the acute stage of carbon monoxide poisoning, diffusion MR images obtained at b = 1000 s/mm2 revealed high signal intensity lesions in the white matter, consistent with restricted diffusion. Low apparent diffusion coefficient values (0.18–0.34 × 10−3 mm2/s) were noted in the affected white matter regions. Follow-up MR imaging performed 16 days later revealed disappearance of white matter lesions, suggesting that during the acute stage of carbon monoxide poisoning, white matter can be more sensitive than gray matter to ischemia.
The imaging features of carbon monoxide poisoning that have been described have been mainly MR imaging features (1–7). Only two reports are available that include diffusion MR imaging and proton MR spectroscopy of acute, interval, and chronic stages of the condition (8–10). We herein report the case of a patient studied by diffusion MR imaging during the acute stage and by follow-up MR imaging on day 16.
Case Report
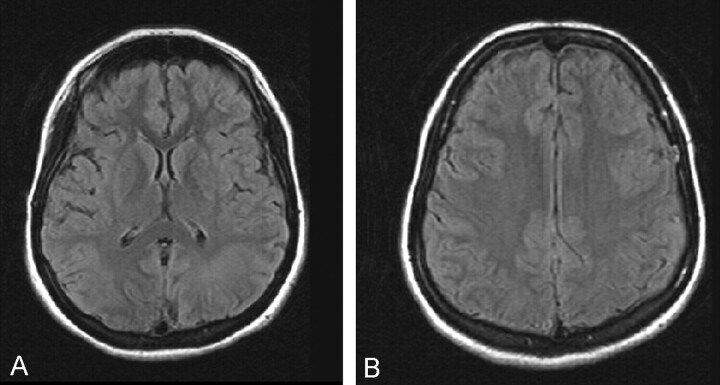
A 9-year-old girl was admitted to the hospital early in the morning in a comatose state that was caused by being in a poorly ventilated room with a coal heater. MR imaging was performed at 1.5 T, including an echo-planar diffusion MR imaging sequence at approximately 12 hours after exposure to carbon monoxide. Findings of fluid-attenuated inversion recovery images were normal (Fig 1A and B).
Fig 1.
MR images obtained during the acute stage of carbon monoxide poisoning.
A, Fluid-attenuated inversion recovery image of the region of the basal ganglia is normal.
B, Fluid-attenuated inversion recovery image corresponding to the images shown in Figure 2A and B appears normal.
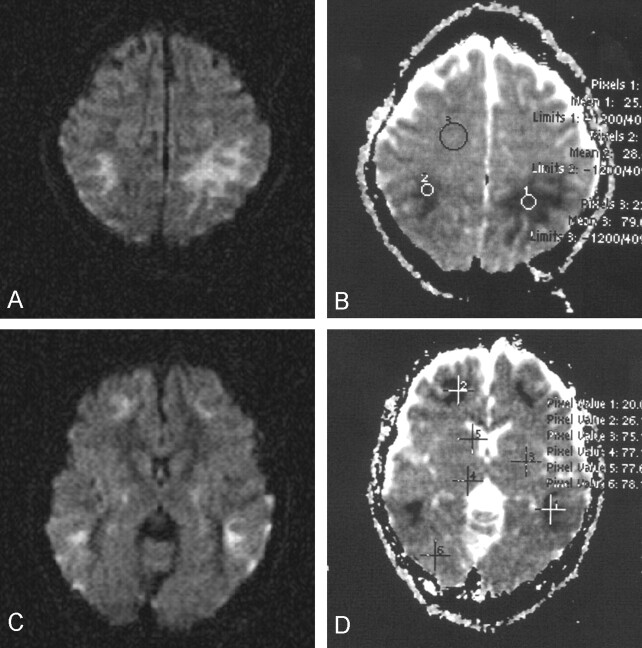
Diffusion MR imaging was performed by using a three-gradient protocol (4000/110 [TR/TE]) Heavily diffusion-weighted (b = 1000 s/mm2) images and automatically generated apparent diffusion coefficient (ADC) maps were studied. ADC values were calculated by electronic readings from ADC maps by variably sized circular region-of-interest evaluations and by pixel-lens evaluations, each containing 16 pixels. On b = 1000 s/mm2 images, widespread symmetrical hyperintense changes were evident and involved mainly the subcortical white matter in both hemispheres, consistent with restricted diffusion (Fig 2A and C). ADC maps revealed low ADC values ranging from 0.18 to 0.34 × 10−3 mm2/s at the corresponding regions (Fig 2B and D). ADC values of unaffected regions of the white matter ranged from 0.73 to 0.82 × 10−3 mm2/s (Fig 2B and D). ADC values from the surrounding cortices were normal, similar to those of unaffected white matter.
Fig 2.
Diffusion MR images obtained during the acute stage.
A, b = 1000 s/mm2 image reveals prominent high signal intensity in the parietal white matter, consistent with restricted diffusion, hence cytotoxic edema. Subtle involvement of outer cortical regions is noted.
B, ADC map (same section as that shown in A) reveals very low ADC values in the affected white matter (0.25 and 0.28 × 10−3 mm2/s; mean values of pixels 1 and 2), compared with the normal ADC value from unaffected white matter (0.79 × 10−3 mm2/s; mean value of pixel 3).
C, b = 1000 s/mm2 image reveals high signal intensity in the frontal and temporal white matter. Some involvement of the posterior portions of the globi pallidi and of the temporal cortices is noted.
D, ADC map (same section as that shown in C) reveals very low ADC values in the affected white matter regions (0.20 and 0.26 × 10−3 mm2/s; mean values of pixels 1 and 2). Normal ADC values from several regions including the basal ganglia, thalamus, and occipital parenchyma are shown (0.75, 0.77, 0.77, and 0.78 × 10−3 mm2/s; mean values of pixels 3–6).
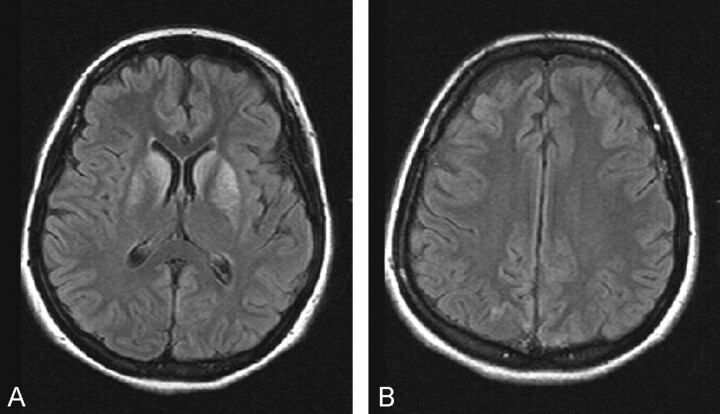
The basal ganglion, thalamus, periventricular white matter, and hippocampus were unaffected. Subtle abnormal signal intensity was seen, however, and was noted to be confined to the posterior portions of the globi pallidi. Frontal and temporal subcortical white matter was involved (Fig 2C). The patient was treated with 100% hyperbaric oxygen, and cardiotonics were applied. Follow-up MR imaging performed 16 days later revealed extensive involvement of the basal ganglion structures (Fig 3A). Interestingly, no remaining white matter lesions were shown (Fig 3B).
Fig 3.
MR images obtained 16 days later.
A, Fluid-attenuated inversion recovery image corresponding to the image shown in Figure 1A reveals development of prominent high signal intensity lesions in the basal ganglia.
B, Fluid-attenuated inversion recovery image corresponding to the image shown in Figure 1B continues to show no white matter abnormalities.
Discussion
With carbon monoxide poisoning, formation of carboxyhemoglobin in the blood results in hypoxia-anoxia and the direct toxic effect of carbon monoxide on mitochondria interferes with oxidative phosphorylation. These lead to anoxic-ischemic encephalopathy, usually with bilateral lesions (1–7). It has been reported that the basal ganglia structures, globus pallidus, putamen, and caudate nucleus are most commonly affected by ischemic lesions. Also, thalamus, periventricular and subcortical white matter, corpus callosum, cerebral cortex, and hippocampus of the temporal lobe may be involved (1–7).
Three recent reports dealt with acute, interval, and chronic stages of carbon monoxide poisoning (8–10). Chalela et al (8) reported one case of carbon monoxide poisoning in a retrospective case study of seven patients with anoxic-ischemic encephalopathy due to a variety of causes, including cardiac arrhythmia myocardial infarction, drug overdose, respiratory failure, and sepsis. All these patients revealed preferential involvement of the white matter covering periventricular white matter, corpus callosum, internal capsule, and the subcortical white matter in a symmetrical fashion (8). Diffusion MR imaging confirmed the presence of restricted diffusion with low ADC values in these regions. Cortex was either less affected or unaffected. On the basis of diffusion MR imaging findings, Chalela et al concluded that prominent, symmetric restricted diffusion can occur early after anoxic-ischemic encephalopathy in the white matter, whereas gray matter involvement may be less prominent.
In the case of carbon monoxide poisoning reported herein, the diffusion MR imaging findings obtained approximately 12 hours after exposure were similar to those reported by Chalela et al (8) in that symmetrical prominent involvement of the white matter was evident with high signal intensity on b = 1000 s/mm2 images, reflecting restriction of mobility of water molecules, hence cytotoxic edema. On corresponding ADC maps, low signal intensity and low ADC values were noted at these regions. Interestingly, the perilesional cortices had only subtle lesions, and no basal ganglion lesion was found. These findings suggested that during the early (acute) stage of carbon monoxide poisoning, the white matter can be more sensitive to ischemia than previously thought. Our experience with this case suggested that diffusion MR imaging is superior to MR imaging with respect to lesion detection (and mathematical information with ADC maps) during the acute stage of carbon monoxide poisoning.
Another study reported the case of a patient with the interval form of carbon monoxide poisoning from the 23rd to 118th day of exposure (9). Low ADC values persisted until the 38th day (23–38 days = interval stage). By the chronic stage (118th day), ADC values had gradually increased, becoming those of macrocystic encephalomalacia (9).
Recently, Teksam et al (10) reported two cases of acute, interval, and chronic stages of carbon monoxide poisoning that initially revealed a restricted diffusion pattern in the white matter. White matter lesions were shown to resolve by 10 months. The authors therefore suggested that this was related to reversible demyelination (10). In the case reported herein, we interestingly noted that the initial white matter lesions seen during the acute stage (Fig 2) almost completely resolved within 16 days and, instead, prominent basal ganglion lesions developed (Fig 3).
These findings suggested that ischemia in the white matter was reversible and that an irreversible type of ischemia gradually developed in the deep gray matter structures over 16 days.
Conclusion
Diffusion MR images obtained at b = 1000 s/mm2 revealed symmetrical high signal intensity white matter changes at approximately 12 hours after exposure to carbon monoxide. This pattern reflected restricted diffusion, hence cytotoxic edema. On ADC maps, low signal intensity and low ADC values were noted. Follow-up MR imaging performed 16 days later revealed extensive basal ganglion involvement without presence of white matter lesions. These findings suggested that during the early stage of carbon monoxide poisoning, white matter may be more sensitive than gray matter to ischemia and ischemia can be reversible. Basal ganglion changes can develop at a later stage. Application of diffusion MR imaging seems to be justified during the early stage of carbon monoxide poisoning.
References
- 1.O’Donnell P, Buxton PJ, Pitkin A, Jarvis LJ. The magnetic resonance imaging appearances of the brain in acute carbon monoxide poisoning. Clin Radiol 2000;55:273–280 [DOI] [PubMed] [Google Scholar]
- 2.Muller NG, Gruber O. High-resolution magnetic resonance imaging reveals symmetric bitemporal cortical necrosis after carbon monoxide intoxication. J Neuroimaging 2001;11:322–325 [DOI] [PubMed] [Google Scholar]
- 3.Gottfried JA, Chatterjee A. Carbon monoxide-mediated hippocampal injury. Neurology 2001;57:17. [DOI] [PubMed] [Google Scholar]
- 4.Schils F, Cabay JE, Flandroy P, Dondelinger RF. Unusual CT and MRI appearance of carbon monoxide poisoning. JBR-BTR 1999;82:13–15 [PubMed] [Google Scholar]
- 5.Gallerani M, La Cecilia O, Serra A, et al. Parkinsonian syndrome after acute carbon monoxide poisoning. Am J Emerg Med 2000;18:833–834 [DOI] [PubMed] [Google Scholar]
- 6.Anton M, Alcaraz A, Rey C, Concha A, Fernandez J. Acute hydrocephalus in carbon monoxide poisoning. Acta Paediatr 2000;89:361–364 [PubMed] [Google Scholar]
- 7.Pavese N, Napolitano A, De Iaco G, et al. Clinical outcome and magnetic resonance imaging of carbon monoxide intoxication: a long-term follow-up study. Ital J Neurol Sci 1999;20:171–178 [DOI] [PubMed] [Google Scholar]
- 8.Chalela JA, Wolf RL, Maldjian JA, Kasner SE. MRI identification of early white matter injury in anoxic-ischemic encephalopathy. Neurology 2001;56:481–485 [DOI] [PubMed] [Google Scholar]
- 9.Murata T, Kimura H, Kado H, et al. Neuronal damage in the interval form of CO poisoning determined by serial diffusion weighted magnetic resonance imaging plus 1H-magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry 2001;71:250–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teksam M, Casey SO, Michel E, Liu H, Truwit CL. Diffusion-weighted MR imaging findings in carbon monoxide poisoning. Neuroradiology 2002;44:109–112 [DOI] [PubMed] [Google Scholar]