Abstract
Summary: We report a case of serologically proved West Nile virus encephalitis. Striking bilateral hyperintensities were noted in the basal ganglia and thalami on T2-weighted and fluid-attenuated inversion recovery MR images, similar to previous accounts of the imaging findings in cases of Japanese encephalitis. Recognition of the MR imaging appearance of this entity is important because of the expanding epidemic.
West Nile virus, a single-stranded RNA flavivirus and member of the Japanese encephalitis virus antigenic complex, was first isolated in 1937 in the West Nile province of Uganda from the blood of a febrile woman (1). Although one of the most disseminated of all of the arboviruses in the Eastern Hemisphere, West Nile virus first emerged in North America in 1999 and caused 62 cases of meningoencephalitis and seven deaths (2). In the subsequent 2 years, the mosquito-borne epidemic spread throughout the mid Atlantic states and began marching into the South and Midwest (3). By 2002, >1600 cases had been reported in an outbreak involving 42 states and the District of Columbia (4).
Imaging studies, especially those including MR imaging, have aided in diagnosing the intracranial manifestations and complications of several viral encephalitides, such as herpes simplex and cytomegalovirus. Multiple articles also have outlined imaging findings for other members of the flavivirus family, such as St. Louis encephalitis and Japanese encephalitis (5–8). Although a few clinical review articles have mentioned imaging findings associated with West Nile virus (2, 9–12), no reports have provided representative images or have identified basal ganglia or thalamic findings. The recent outbreak of West Nile virus has emphasized the increasing importance of recognizing any imaging findings that could aid diagnosis. We present MR imaging findings of a woman with serologically proved West Nile virus encephalitis.
Case Report
A 54-year-old woman presented to our emergency department with a 4-day history of fevers, abdominal pain, nausea, emesis, myalgias, arthralgias, and headache and a recently resolved rash. The patient’s mental status had progressively deteriorated with bouts of confusion, disorientation, and dysarthria as well as intermittent aphasia. At presentation, the patient was somnolent, unable to follow simple commands, and had receptive aphasia. The remainder of the patient’s examination revealed no focal neurologic signs.
CSF showed 71 nucleated cells consisting of lymphocytes (87%), neutrophils (8%), and macrophages (5%). The CSF protein and glucose concentrations were 63 and 53 mg/dL, respectively. Serum glucose was 117 mg/dL at the time of the lumbar puncture.
Results of cranial CT were reported to be normal, although in retrospect, subtle edema symmetrically involved the thalami, lentiform nuclei, and caudate nuclei (Fig 1A). Empiric antiviral therapy and antibiotics were instituted with the presumptive diagnosis of viral meningoencephalitis. The patient’s condition slightly improved initially, and she was able to follow commands. By the 4th hospital day, the patient’s condition had deteriorated with the development of stupor, aphasia, and severe paresis of both lower extremities and, to a lesser degree, bilateral upper extremities. The results of nerve conduction studies were negative. These clinical findings were highly suspicious for West Nile virus, based on well-documented cases of paresis and flaccid paralysis in the outbreak of 1999 in New York City (2, 13). CSF tests failed to reveal evidence of herpes simplex virus, enterovirus, and cytomegalovirus, and bacterial cultures, acid-fast bacterial cultures, and cryptococcus preparations were also negative. West Nile virus- arbovirus panels were pending at the time that MR imaging of the brain was requested for further evaluation.
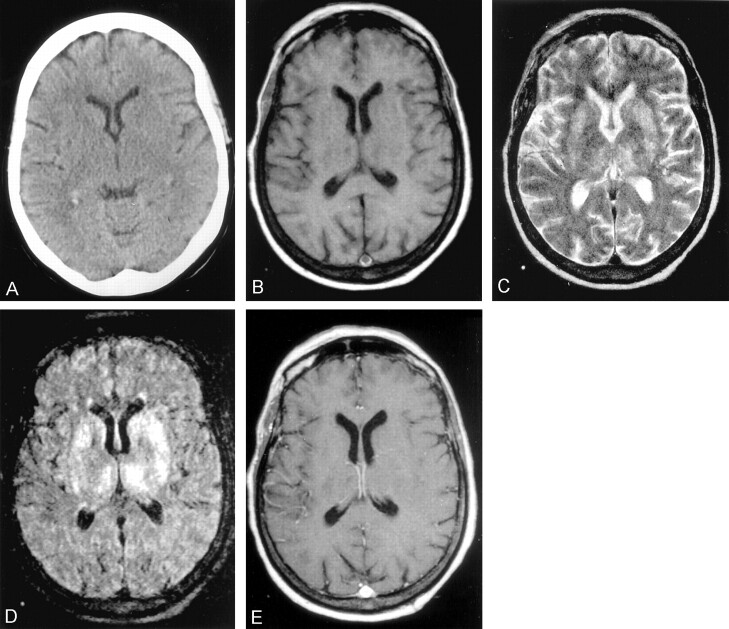
Fig 1.
Images from the case of a 54-year-old woman with serologically proved West Nile virus encephalitis.
A, Unenhanced CT scan of the brain shows subtle bilateral swelling of the basal ganglia and thalami manifested by symmetric hypoattenuation and loss of the anatomic definition of these structures.
B, Unenhanced axial spin-echo T1-weighted MR image (560/17 [TR/TE]) shows uniformly isointense basal ganglia and thalami without the usual anatomic definition of these structures.
C, Axial fast spin-echo T2-weighted MR image (3840/99) shows bilateral hyperintensity of the caudate nuclei, putamina, and thalami. The left side is more involved than the right side, and the globus pallidi are relatively spared.
D, Axial fluid-attenuated inversion recovery MR image (9999/119; inversion time, 2389 ms) reveals bilateral markedly hyperintense basal ganglia and thalami, predominantly on the left side.
E, Contrast-enhanced axial T1-weighted MR image (560/17) fails to show enhancement of the deep gray matter structures.
MR imaging of the brain (Fig 1B-E) revealed abnormal bilateral hyperintensity of the thalami, caudate nuclei, and lentiform nuclei on fluid-attenuated inversion recovery and T2-weighted images. No abnormal signal intensity or enhancement was noted on T1-weighted images. Diffusion-weighted imaging was unavailable. Serologic testing subsequently secured the diagnosis of West Nile virus with detection of immunoglobulin M antibody in the serum and a positive plaque reduction neutralization test, the latter being highly specific for West Nile virus.
Supportive care was continued, and the patient progressively improved with partial recovery of motor and language function and was discharged to a rehabilitation center for further management. She continued to improve and was discharged. She failed to return for her outpatient visit, and no follow-up images were obtained.
Discussion
Infection with West Nile virus typically causes an influenza-like febrile illness that may progress to encephalitis, meningitis, and polyradiculitis in a minority of patients (9). A variety of birds serve as principal reservoirs of the virus, and transmission is accomplished by bird-feeding mosquitos. Man and other vertebrates are incidental hosts (9).
Although West Nile virus was virtually unknown in the Western hemisphere until a few years ago, the number of documented cases in the United States markedly climbed in 2002. The health risk of the epidemic will likely expand in 2003. Serologic surveys performed in the United States and Romania indicate that severe complications are infrequent and that only 0.7% of infections result in neurologic sequelae (14, 15). Nevertheless, the disease can be fatal, especially in the elderly. With >1600 cases of meningoencephalitis reported in 2002, the number of persons actually infected probably approached 200,000 persons (4). Although the current mainstay of treatment is supportive, some institutions recommend quickly initiating treatment with interferon-α2b in severe cases based on in vitro studies indicating that high doses were efficacious against the West Nile virus (16).
Imaging could play an especially important role in the future, considering that current serologic testing is an extremely lengthy process requiring confirmation by the Centers for Disease Control. Some authors have superficially commented on the imaging findings of West Nile virus in clinical reviews of the disease (2, 9–11). For example, Nash et al (2) noted that the CT findings of 43 patients were normal and that the MR images of five of 16 patients showed enhancement of the leptomeninges or periventricular regions. These authors, however, failed to present images of or note abnormalities of the deep gray matter structures (2, 9). We present a single case of serologically proved West Nile virus with MR imaging findings of bilateral hyperintensity within the thalami, caudate nuclei, and lentiform nuclei on fluid-attenuated inversion recovery and T2-weighted MR images. The nearly symmetrical involvement seen in this case is an unusual imaging feature not ordinarily identified in association with infectious or inflammatory processes.
Other investigators have reported similar findings with other flaviviruses, such as Japanese encephalitis (6, 7). Correlated neuropathologic data from four fatalities resulting from West Nile virus in 1999 include microglial nodules composed mainly of lymphocytes and histiocytes and variable mononuclear perivascular inflammation most consistently involving the thalamus and medulla (17). The distribution and appearance of the MR imaging findings in our case fit well with the known pathologic information regarding this disease. The explanation for the symmetrical involvement of the deep gray matter structures by some members of the flavivirus family is unknown. Inherent metabolic activity levels and vascular supplies of these structures may play a role. Discovered autoantibodies to myelin basic protein and neurofilaments in some cases of Japanese encephalitis raise the possibility of a superimposed immunologic component to these diseases that may selectively involve certain portions of the CNS (7).
Unlike other reported cases, our case showed no meningeal or periventricular enhancement. This apparent discrepancy in reported imaging findings from various series might be due to differential involvement of brain and meninges in the patients examined. Serial images of large groups of patients will be useful in evaluating the evolution of imaging findings for these patients. Although not seen in our case, the varying degrees of white matter involvement on images reported by some authors may also be influenced by the prevalence of incidental small vessel atherosclerotic disease commonly encountered in elderly patients who are also at risk for neurologic complications of West Nile virus. Whether West Nile virus involvement of the basal ganglia consistently alters MR signal intensity patterns and to what degree West Nile virus resembles other flaviviruses in MR imaging appearance remain to be proved. Nevertheless, the recognition of MR imaging features of the intracranial manifestations of West Nile virus will aid in the diagnosis of a disease that often presents with a protean clinical picture.
Conclusion
In this case of serologically proved West Nile virus encephalitis, bilateral hyperintensities were observed in the basal ganglia and thalamus on T2-weighted and fluid-attenuated inversion recovery MR images, resembling the imaging findings previously reported in Japanese encephalitis. Recognition of these MR imaging findings of West Nile virus encephalitis is important because of the growing epidemic.
Footnotes
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Defense.
References
- 1.Smithburn KC, Hughes TP, Burke AW, Paul JH. A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med Hyg 1940;20:471–492 [Google Scholar]
- 2.Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in New York City area in 1999. N Engl J Med 2001;344:1807–1814 [DOI] [PubMed] [Google Scholar]
- 3.West Nile virus activity: United States, 2001. MMWR Morb Mortal Wkly Rep 2002;51:497–501 [PubMed] [Google Scholar]
- 4.West Nile virus activity: United States, September 12–18, 2002, and Ohio, January 1-September 12, 2002. MMWR Morb Mortal Wkly Rep 2002;51:836–837 [PubMed] [Google Scholar]
- 5.Cerna F, Mehrad B, Luby JP, Burns D, Fleckenstein JL. St Louis encephalitis and the substantia nigra: MR evaluation. AJNR Am J Neuroradiol 1999;20:1281–1283 [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, Misra UK, Kalita J, Salwani V, Gupta RK, Gujral R. MRI in Japanese encephalitis. Neuroradiology 1997;39:180–184 [DOI] [PubMed] [Google Scholar]
- 7.Abe T, Kojima K, Shoji H, et al. Japanese encephalitis. J Magn Reson Imaging 1998;8:755–761 [DOI] [PubMed] [Google Scholar]
- 8.Shoji H, Kida H, Hino H, et al. Magnetic resonance imaging findings in Japanese encephalitis; white matter lesions. J Neuroimaging 1994;4:206–211 [DOI] [PubMed] [Google Scholar]
- 9.Meek J. West Nile virus in the United States. Curr Opin Pediatr 2002;14:72–77 [DOI] [PubMed] [Google Scholar]
- 10.Chowers MY, Lang R, Nassar F, et al. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg Infect Dis 2001;7:675–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss D, Carr D, Kellachan J, et al. Clinical findings of West Nile virus infection in hospitalized patients, New York and New Jersey, 2000. Emerg Infect Dis 2001;7:654–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen LR, Marfin AA. West Nile virus: a primer for the clinician. Ann Intern Med 2002;137:173–179 [DOI] [PubMed] [Google Scholar]
- 13.Ahmed S, Libman R. Guillain-Barré syndrome: an unusual presentation of West Nile virus infection. Neurology 2000;55:144–146 [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Serosurveys for West Nile virus infection: New York and Connecticut counties, 2000. MMWR Morb Mortal Wkly Rep 2001;50:37–39 [PubMed] [Google Scholar]
- 15.Tsai TF, Popovici F, Cernescu C, Campbell GL, Nedelcu NI. West Nile encephalitis epidemic in southeastern Romania. Lancet 1998;352:767–771 [DOI] [PubMed] [Google Scholar]
- 16.Anderson JF, Rahal JJ. Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro [letter]. Emerg Infect Dis 2002;8:107–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sampson BA, Armbrustmacher V. West Nile encephalitis: the neuropathology of four fatalities. Ann N Y Acad Sci 2001;951:172–178 [PubMed] [Google Scholar]