Abstract
BACKGROUND AND PURPOSE: Using perfusion- and diffusion-weighted MR imaging in acute ischemic stroke of the middle cerebral artery (MCA), previous studies have shown a typical pathophysiologic pattern that is characterized by a perfusion deficit larger than the diffusion lesion (mismatch), with the final lesion usually comprising the initial diffusion lesion (core) plus parts of the initial mismatch area. Little is known about underlying pathophysiology in small ischemic stroke. In this study, we used perfusion- and diffusion-weighted MR imaging to investigate the underlying pathophysiology of small subcortical ischemia.
METHODS: Six consecutive patients (age range, 42–76 years) with small subcortical ischemia were examined by using a 1.5-T MR system 2–5, 22–55, and 144–392 hours after the onset of symptoms. T2-weighted, diffusion-weighted imaging at b=0 s/mm2 and b=1000 s/mm2, and bolus-track perfusion-weighted imaging were performed. Lesion sizes were determined on the basis of T2-weighted findings as well as those of apparent diffusion coefficient (ADC) maps and CBF.
RESULTS: In every patient, the initial CBF lesion was smaller than the initial ADC lesion. Both the CBF lesion and the ADC lesion increased in size from first to second examination. In all instances, however, the CBF lesion remained smaller than the ADC lesion. The CBF lesion observed during the acute phase and the one seen on the following days were both smaller than the final T2 lesion.
CONCLUSION: Our data suggest that in contrast to previous findings in MCA ischemia in small subcortical infarcts tissue damage may spread beyond the area of the initial perfusion disturbance. In light of the small number of patients, further studies will have to address the relevance of this observation.
Pathophysiologic events in acute ischemic stroke are extremely heterogeneous among patients. Furthermore, in each patient, pronounced pathophysiologic changes occur during the first hours and days after the onset of cerebral ischemia. Knowledge about these events would be important for tailoring therapy (eg, thrombolysis) to the individual patient. One way to obtain this information in individual subjects is with perfusion- and diffusion-weighted MR imaging. Both perfusion- and diffusion-weighted imaging allow for a very sensitive detection of acute ischemic stroke within the first hours after the onset of symptoms. Whereas perfusion-weighted imaging allows for a delineation of the perfusion disturbance, signal intensity increases at diffusion-weighted imaging, corresponding to a decrease of the apparent diffusion coefficient (ADC), are generally thought to reflect cytotoxic edema (1–4).
In patients in whom arterial occlusion persists at the time of examination, the area of perfusion disturbance is usually larger than the area of reduced ADC (“mismatch”). The lesion detected at diffusion-weighted imaging has been discussed as the core of the ischemic area, indicating tissue with a high probability to become infarcted, although reversible diffusion lesions have been reported (5), and in animal studies, the outer rim of the diffusion lesion has been described as part of the penumbra (6). The area of decreased ADC may further enlarge, and in most cases this occurs within the mismatch area (7–10). Hence, it was suggested that the mismatch region may include the ischemic penumbra (11), although the terms “mismatch” as an operational definition comparing two MR imaging parameters and “penumbra” do not necessarily coincide. In patients in whom reperfusion has occurred, the diffusion-weighted lesion was either of the same size or larger than the perfusion-weighted lesion and usually no further lesion enlargement was observed at diffusion-weighted imaging (8).
These rather typical observations of underlying pathophysiology in ischemic stroke are derived from studies in which most patients had territorial infarction usually affecting the middle cerebral artery (MCA) or its major branches. The situation in these cases is characterized by potentially significant collateral circulation due to meningeal collaterals or collateral circulation within the circle of Willis.
This pathophysiologic constellation, however, may not necessarily apply to all types of ischemic stroke. Different brain areas are characterized by variable anatomic organization of collateral arterial supply and the susceptibility of cerebral tissue to ischemia differs among the various anatomic brain regions. Therefore, it is not justified to assume that the underlying pathophysiology always follows the same “rules,” but rather studies on different types of ischemic stroke are warranted. In the present study, by using perfusion-, diffusion-, and T2-weighted imaging, we investigated perfusion and subsequent infarction patterns in six consecutive patients with ischemic stroke in a small subcortical artery, either the anterior choroidal artery (AChA) or small lenticulostriate branches of the MCA.
Methods
Patients
Since April 1998, we prospectively recruited patients with acute ischemic stroke (<6 hours after symptom onset) for perfusion- and diffusion-weighted studies. For this study, we sought patients with ischemia in the AChA territory, which we operationally defined as described below. Between April 1998 and October 2000, eight such patients were admitted to our hospital within 6 hours after the onset of symptoms. Of these patients, one refused to participate in the study, and in another case, initial MR imaging was performed but no follow-up study could be performed. The remaining six patients (three female, three male; mean age, 63 years; age range, 42–76 years) were included in this study. MR imaging examinations took place 2–5, 22–55, and 144–392 hours after the onset of symptoms.
Our operational definition of the AChA territory followed Damasio’s template of cerebral vascular territories (12) and the classificiation of subcortical infarction as given by Donnan et al (13). As inclusion criteria, we required the posterior limb of the internal capsule and the medial globus pallidus to be affected. It should be noted, however, that, whereas we saw involvement of medioposterior parts of the lentiform nucleus in all patients, in some of the patients it was difficult to separate involvement of medial globus pallidus versus posterior putamen, the latter being part of the lenticulostriate territory.
Furthermore, it is controversial whether the paraventricular corona radiata belongs to the AChA as well. On the one hand, the study by Mohr et al (14) suggested that it does not belong to the AChA territory, but, on the other hand, work by other authors (15, 16) indicate that it is frequently involved in AChA infarcts. Because of this controversy, our operational definition of AChA infarcts (see above) did not require the paraventricular corona radiata to be involved. It is interesting, however, that we found it to be affected in all of our patients. In addition to the lesion sites discussed above, which were affected in all patients, in three patients the medial temporal lobe and in one patient the posterior thalamus were also part of the ischemic lesion. These are areas that have been described as variably belonging to the AChA territory in previous studies (14, 17).
In view of the controversies about the AChA territory and the lack of angiographic confirmation in our study, in this report we refer to the infarcts as small subcortical ischemia (and not AChA infarcts). Figure 1 demonstrates the anatomic distribution of the infarct in one of our patients (patient 1, 392 hours after the onset of symptoms).
Fig 1.
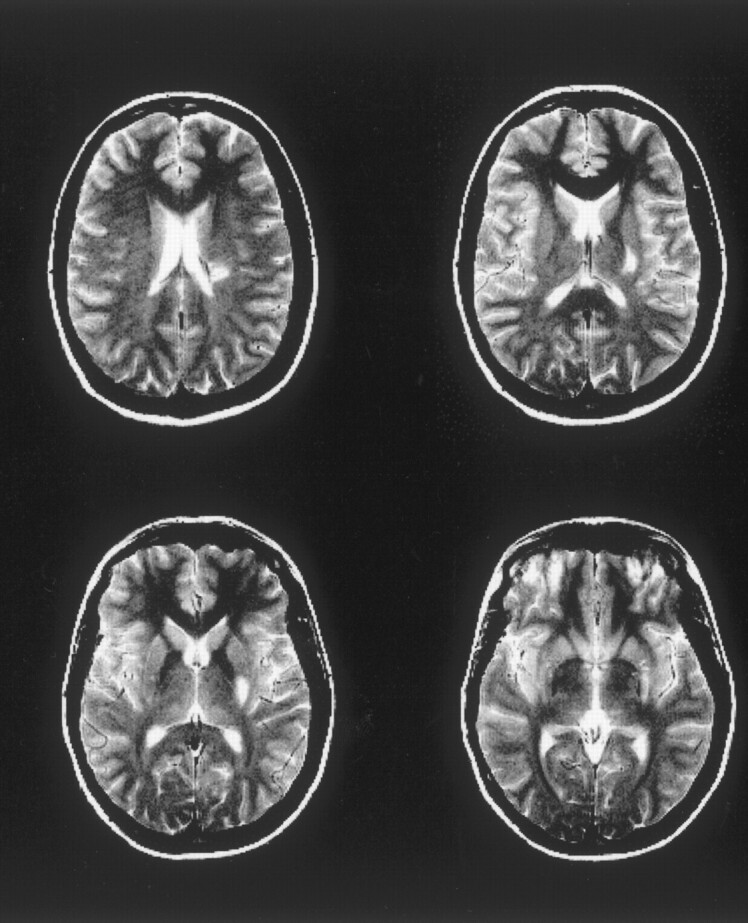
Patient 1. Whole lesion, as assessed by T2-weighted imaging at the third examination, 392 hours after the onset of symptoms. This demonstrates the typical lesion location in our patients, in all cases affecting the posterior limb of the internal capsule and the medial globus pallidus. In addition, the corona radiata is involved.
MR Imaging Measurements
MR imaging measurements were performed on a 1.5-T Vision MR imager (Siemens Medical Systems, Erlangen, Germany). For T2-weighted imaging, a multi-echo turbo spin-echo sequence (TR/TE, 2900/15, 75, and 135; matrix, 256 × 256; field of view, 240 mm; section thickness, 6 mm; intersection gap, 0.6 mm) was employed. Spin-echo diffusion-weighted echo-planar imaging (4657/118; matrix, 128 × 128; field of view, 240 mm; section thickness, 6 mm; intersection gap, 0.6 mm; diffusion gradients in three orthogonal directions) was performed by using two different b values (0 s/mm2 and 1000 s/mm2). Twenty transverse sections in diffusion- and T2-weighted imaging had the same section location in the same plane in anteroposterior commissure orientation.
Perfusion-weighted imaging was performed acutely and subacutely by using T2*-weighted echo-planar sequences (1000/54; matrix, 128 × 128; field of view, 240 mm; section thickness, 6 mm; intersection gap, 0.6 mm). Six transverse sections always covered the infarcted area and had the same section location in the same plane in anteroposterior commissure orientation, corresponding to sections in diffusion-weighted imaging. Twenty milliliters of Magnevist (gadopentetate dimeglumine; Schering, Berlin, Germany) followed by 20 mL saline were injected intravenously at a rate of 4 mL/s by using a power injector (Spectris; Medrad). Data acquisition started at the beginning of the contrast agent injection with a temporal resolution of 1 second and was continued for 60 seconds.
Data Analysis
Perfusion- and diffusion-weighted data were postprocessed with algorithms developed by the authors by using IDL 5.0 software (Research Systems Inc., Boulder, CO). Pixel-based calculation of mean transit time (MTT) was performed by using the normalized first moment of the logarithmic signal intensity time course (18, 19). The “zeroeth” moment of the logarithmic signal intensity time course is proportional to cerebral blood volume (CBV) (20). Using this approach, only relative CBV values can be determined; CBF values determined as the ratio of CBV to MTT are also relative values. ADC was calculated for each direction of diffusion-weighted imaging and added to obtain the trace.
Three independent, blinded observers (C.A.D., C.M.K., B.I.R.) determined lesion volumes by using the software program NIH Image (National Institutes of Health, Bethesda, MD). To overcome the limitations of the frequently used manual determination of lesion size, a standardized method for quantitative volumetric studies was employed. First, for each lesion observed on CBF maps, ADC maps, and T2-weighted images that were contralateral mirror regions of interest were manually generated as control regions. On the basis of the mean signal intensity of this control region, a threshold was set for definition of the ischemic lesion on the affected side.
Two thresholds were employed for determination of the CBF lesion volume, 54.3% (1) and 75% (2). The 54.3% threshold was derived from a recent positron emission tomography (PET) study that had determined the upper and lower CBF limits of the ischemic penumbra to be at 54.3% and 22.0% of CBF of the contralateral site (in that study, these values represented the negative and positive 95% prediction limits for subsequent infarction, respectively [21]). To detect potentially minor perfusion disturbances, we chose a second threshold at 75% (25% perfusion reduction), which determined all regions with any measurable decrease of CBF.
The threshold for increased intensity on T2-weighted images was set at the mean signal intensity of the control region on T2-weighted images +2 SD and for decreased ADC at the mean ADC of the control region−2 SD. The threshold ADC values for a decreased ADC obtained with this method were within the range of data from animal (22–25) as well as human (26) studies. Both thresholds resulted in lesion sizes similar than those obtained by manual delineation of the pathologic areas. Both values also are conservative, because they seem more likely to underestimate than to overestimate the “true” lesion size. The lesion volumes as given in the Table represent the mean values ± SD resulting from individual measurements of the three observers.
Summary of lesion volumes for CBF (thresholds of 54.3% and 75%), ADC, and T2-weighted imaging
| Age/Sex | Time (h) | CBF (54.3%) | CBF (75%) | ADC | T2 | |
|---|---|---|---|---|---|---|
| 1 | 42/F | 4 | 62 ± 35 | 116 ± 23 | 1914 ± 121 | 1489 ± 140 |
| 55 | 1055 ± 40 | 1461 ± 23 | 2562 ± 54 | 2734 ± 17 | ||
| 392 | – | – | 426 ± 27 | 1929 ± 137 | ||
| 2 | 66/F | 5 | 95 ± 32 | 506 ± 32 | 1427 ± 92 | 364 ± 51 |
| 29 | 162 ± 23 | 603 ± 23 | 1756 ± 155 | 842 ± 36 | ||
| 321 | – | – | 1368 ± 106 | 1910 ± 54 | ||
| 3 | 76/F | 5 | 158 ± 26 | 169 ± 40 | 510 ± 15 | 205 ± 46 |
| 22 | 405 ± 40 | 509 ± 40 | 1197 ± 176 | 559 ± 37 | ||
| 209 | – | – | 1364 ± 159 | 2174 ± 96 | ||
| 4 | 64/M | 3 | 201 ± 35 | 386 ± 35 | 889 ± 151 | 441 ± 52 |
| 28 | 348 ± 23 | 542 ± 35 | 1616 ± 47 | 1678 ± 60 | ||
| 148 | – | – | 2754 ± 109 | 2987 ± 27 | ||
| 5 | 60/M | 5 | 518 ± 35 | 1105 ± 35 | 2564 ± 27 | 1118 ± 63 |
| 24 | 882 ± 23 | 1322 ± 23 | 3049 ± 23 | 2862 ± 41 | ||
| 144 | – | – | 2645 ± 236 | 3312 ± 27 | ||
| 6 | 71/M | 2 | 423 ± 23 | 1076 ± 35 | 2101 ± 75 | 741 ± 54 |
| 29 | 896 ± 13 | 1686 ± 35 | 3288 ± 58 | 2877 ± 41 | ||
| 220 | – | – | 2521 ± 27 | 4342 ± 44 |
Note.—Lesion volumes are given in mm3 (Mean ± SD from measurements by three independent observers).
Results
For each subject, lesion volumes determined from CBF maps, ADC maps, and T2-weighted images are given in the Table. In each subject, the size of the CBF lesion (at both thresholds) at the first examination was smaller than the size of the ADC lesion (P < .005 at the threshold of 54.3%, P < .006 at the threshold of 75% [paired t test]). The latter was larger than the acute T2 lesion in all subjects (P < .010 [paired t test]); however, it was smaller than the final T2 lesion (P < .022 [paired t test]), except for in patient 1, in whom both had approximately the same size.
During the second examination, the size of the CBF lesions (at both thresholds) had increased in all subjects (P < .038 at the threshold of 54.3%, P < .060 at the threshold of 75% [paired t test]); however, the CBF lesions were still smaller than the ADC lesions (P < .001 at the threshold of 54.3%, P < .001 at the threshold of 75% [paired t test]), which had also increased in size in all subjects (P < .002 [paired t test]).
When comparing the size of the CBF lesions during the first and second examination with the size of the T2 lesion determined at the final examination, the CBF lesions (at both thresholds) were always smaller than the final T2 lesion in each subject (threshold of 54.3%: P < .001 at first examination, P < .002 at second examination, [paired t test]; threshold of 75%: P < .001 at first examination, P < .003 at second examination [paired t test]) .
At the second examination in four patients (2, 3, 5, 6), the ADC lesion was larger than the T2 lesion. In patient 4, no significant difference in lesion size was observed, whereas in patient 1 the ADC lesion was smaller than the T2 lesion. The CBF lesion at the second examination was smaller than the T2 lesion (P < .008 at the threshold of 54.3%, P < .015 at the threshold of 75% [paired t test]) in all subjects except patient 3, in whom both lesions were approximately the same size.
The mean ADC values within the lesion at the first examination were 5.4 ± 0.4 × 10−4 mm2/s and at the second examination 5.3 ± 0.5 × 10−4 mm2/s (mean ± SD). In all patients, no significant change in ADC values was observed between the first and the second examination. Normal values for ADC were between 8.1 and 10.3 × 10−4 mm2/s, in agreement with previously reported values (eg, by Uluğ et al [27]).
The main points are illustrated in Figures 2, 3, and 4. Figure 2 gives CBF and ADC maps and the corresponding final T2-weighted image obtained in patient 6. Figure 3 displays perfusion and ADC maps obtained in patient 1.
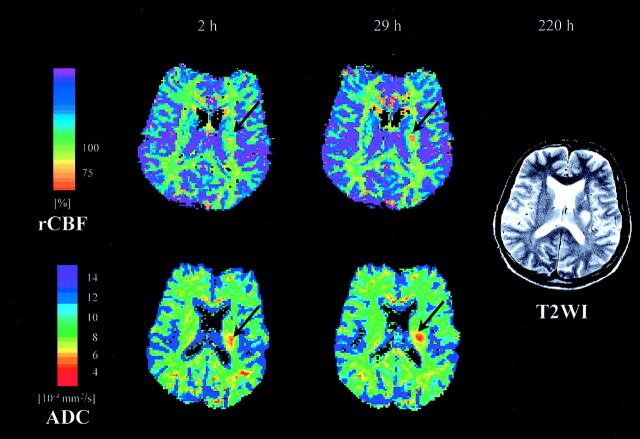
Fig 2.
CBF maps (top, relative CBF units, threshold 75% of contralateral side) and ADC maps (bottom) are given for patient 6 at 2 hours and 29 hours after symptom onset, respectively. Right, the corresponding T2-weighted image obtained at the final examination after 220 hours. The CBF lesion (arrow) increases in size from first to second examination. At both time points, the CBF lesion is smaller than the ADC lesion (arrow) and smaller than the final T2 lesion.
Fig 3.
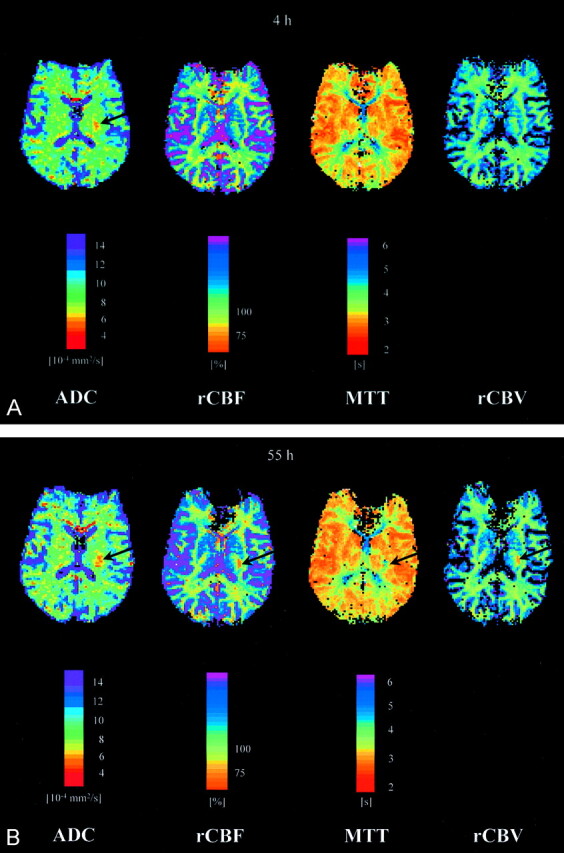
A, ADC, CBF (relative CBF units, threshold 75% of contralateral side), MTT, and CBV maps 4 hours after onset of symptoms, for patient 1. None of the three different types of perfusion maps (MTT, CBF, CBV) showed any pathologic changes (on adjacent sections, however, small areas of altered perfusion were detected in this patient), whereas in the ADC map an ischemic area (arrow) was seen. B, ADC, CBF (relative CBF units, threshold 75% of contralateral side), MTT, and CBV maps 55 hours after onset of symptoms, for patient 1. The three different types of perfusion maps indicate an area of altered perfusion (arrows) smaller than the ADC lesion (arrow) at the same time point.
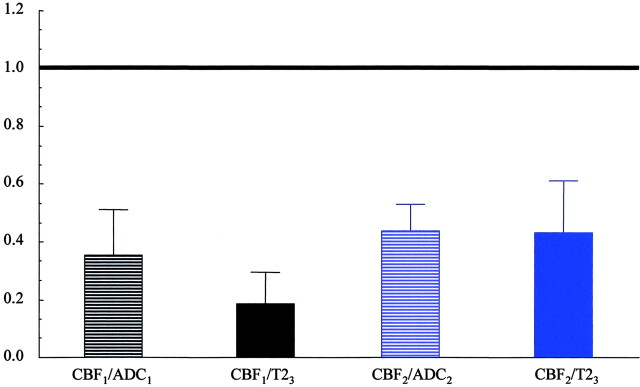
Fig 4.
Each column in this figure indicates the ratio (mean across all patients) of two lesion sizes. CBF lesion volumes are given at the threshold of 75%. The first and second columns (black) give the ratios CBF1 to ADC1 and CBF1 to T23. The CBF lesion at first examination is smaller than the ADC lesion at the same time point (P < .006, paired t test) as well as the final T2 lesion (P < .001, paired t test) as indicated by the ratio below unity. The third and fourth column (blue) give the ratios of the CBF lesion at the second time point to the ADC lesion at the same time point as well to the final T2 lesion. The ratio below unity indicates that the CBF lesion in the subacute phase is smaller than the ADC lesion at the same time point and the final T2 lesion (P < .001, P < .003, paired t test).
Figure 4 illustrates the main points of this article by giving ratios (mean across all patients) between key variables. The CBF lesion–ADC lesion ratio is given for the first and the second examination (first and third column). The ratios are smaller than unity, illustrating that at both time points the CBF lesion is smaller than the ADC lesion. The second and fourth ratios, CBF lesion–T2 lesion, are also clearly below unity, illustrating the point that the final T2 lesion is larger than the CBF lesion at first, as well as second, examination.
Discussion
In this study on patients with acute small subcortical ischemia, we report the following main findings: in the first hours after stroke onset, the perfusion deficit was smaller than the area of decreased ADC (1); during the days that follow, both the perfusion and ADC lesion increased in size, the perfusion deficit being always smaller than the ADC lesion (2); the final lesion (judged from the T2 lesion at the last examination) was larger than the area of CBF reduction in the acute phase as well as at the follow-up examination (3).
These findings are strikingly different from what has been reported in many studies in which diffusion- and perfusion-weighted imaging were used (the latter based on either MTT or CBF maps) and which mainly included patients with territorial infarcts in the MCA territory (7, 8, 28, 29). The main difference is that, in previous studies, the final lesion developed inside the area of the initial perfusion reduction, whereas our findings suggest that the lesion may spread beyond the initial perfusion disturbance. Before drawing such a conclusion, however, the issue of validity of our measurement is important. Could it be that we either underestimated the true size of the CBF lesion or overestimated the true size of the ADC lesion? After having performed preliminary manual determinations of lesions sizes, we attempted to measure lesion sizes as objectively as possible, in particular avoiding any underestimation of the CBF lesion and any overestimation of the ADC and T2 lesion sizes.
To determine the size of the ADC lesion, we compared values with the contralateral side and set a threshold at the mean −2 SD. We are aware that the definition of a standard deviation does not necessarily imply physiologic or pathophysiologic relevance of the chosen threshold. In particular, it seems entirely possible that higher values (closer to the mean) may also be pathologic. Therefore, it may very well be that our lesion size is an underestimate of the truly affected brain region. If one assumes that the contralateral ADC values reflects the condition of the affected area before stroke onset, then at least 95% of the pixels have “decreased” ADC values (ie, a possible underestimation of the lesion would be on the order of 5%, at the most). Because the difference in lesion size on CBF maps compared with that of ADC maps far exceeds this percentage, we feel that we safely can assume that the ADC lesion is larger than the CBF lesion (in fact the ratio of CBF lesion [threshold of 75%] to the ADC lesion at the first examination was 0.35 ± 0.16 [mean ± SD]). A similar conclusion can be made for the lesion sizes determined on the basis of T2-weighted findings: the threshold of (contralateral) mean + 2 SD does not guarantee pathophysiologic relevance (ie, increases in signal intensity below this threshold may also signify a pathologic findings for similar reasons similar to those attributable to ADC lesions); however, this threshold most likely results in an underestimation of the true lesion size.
Regarding the CBF lesion, we chose two thresholds: one at 54.3% and one at 75%. The 54.3% threshold was derived from a recent PET study that had determined the upper and lower CBF limits of the ischemic penumbra to be at 54.3% and 22.0% of CBF of the contralateral site (in that study, these values represented the negative and positive 95% prediction limits for subsequent infarction, respectively) (21). We are aware of the difficulties in quantifying bolus-track MR imaging data and that these values do not completely correspond to CBF values obtained in PET studies. It is interesting that a recent literature overview comparing CBF values derived from PET versus those from MR imaging studies revealed overall a good agreement (30–35). We believe that, even if there are some errors when relating MR imaging and PET values, it is still justified to assume a similar order of magnitude as a threshold. As an additional safety margin, we also used another threshold of 75% (25% perfusion reduction), which includes very minor CBF disturbances that supposedly should not be associated with any subsequent damage. We believe that the thresholds we have chosen safely give an outer border of relevant perfusion disturbance and thus, if anything, overestimate the true size of the CBF lesion. We are aware of potential partial volume effects at the border of the small lesions; however, the safety margins illustrated above should exclude any significant influence of such partial volume effects.
Considering that our definition of the diffusion lesion represents an underestimation of the true diffusion lesion and that our definition of the perfusion lesion attempts to represent an overestimation of the true perfusion lesion, it seems safe to make the main point of our study, that the initial perfusion lesion is smaller than the initial diffusion lesion.
Furthermore, our main conclusion, that ischemic damage spreads beyond the initial area of ischemia, does not even depend on the comparison between the diffusion and perfusion lesion. Let us assume any size comparison between diffusion and perfusion lesions is not valid, and let us furthermore assume that there is a sensitivity problem with small lesions with perfusion-weighted imaging. What remains is the comparison between subsequent perfusion- and diffusion-weighted examinations. The initially small perfusion lesion becomes larger in all subjects, and the diffusion lesion also becomes larger in all subjects. Thus, even if the size comparison between perfusion and diffusion lesions were not correct, it seems reasonable to conclude that the ischemic lesion becomes larger in the interval from the first to the second examination. Because our methodology was identical during the first and second examinations, it seems difficult to evoke any methodologic interpretation of this finding. If we now consider studies of large MCA infarction, it is well described that the ADC lesion can become larger between the first and second examination; however, it is not typical that the perfusion lesion becomes larger.
The question remains whether the lesions in our study are simply too small to accurately determine lesion sizes, because there have been reports in the literature based on the relatively poor sensitivity of CT perfusion imaging to detect small lesions (36). Faced with such small lesions, we did everything to account for subjective influences. Thus, we used an objective threshold (discussed above), and lesion sizes were independently determined by three blinded investigators. The variability of their measurements is expressed in the standard deviations given in the Table. Although there clearly is some variation between observers, these variations are small compared with the differences in lesion sizes from which we draw the major conclusions in this report. The fact that in all six subjects the increase in both CBF lesion and ADC lesion size from the first to the second measurement is clearly depicted in the measured volumes may serve as an indirect confirmation of the sensitivity of our measurements of these small lesion volumes. Our pathophysiologic conclusions mainly depend on these comparisons that indicate lesion growth after the first examination. The increase in lesion size within one technique (CBF map and ADC map) will most likely not be affected by further improvements in the precision of the measurements.
In our study, we have employed the diffusion and perfusion imaging approach that is common to most MR imaging studies in acute stroke. Future work on this issue should be able to further improve on the precision of perfusion and diffusion measurements by taking advantage of higher field strengths, diffusion tensor imaging (37), and differentiation of fast and slow diffusion tensors (38).
We believe that, even considering potential sources of errors in our measurements, the main findings of this study are valid. What, then, could this mean in terms of underlying pathophysiology?
As outlined above, in MCA ischemia, usually a mismatch (perfusion > diffusion) is observed, and when the pathophysiologic pattern of a diffusion lesion larger than the perfusion lesion is seen, this is interpreted as a sign of early reperfusion. Thus, the question arises whether early (quick) reperfusion would be consistent with our findings. Because the involved vessels (AChA, lenticulostriate branches) are too small to be clearly evaluated by MR angiography, we cannot answer this question definitively. We believe that our finding of an increase in perfusion size between day 1 and day 2 is an argument against early reperfusion. Studies in which early reperfusion (after thrombolysis) has been monitored have consistently reported a subsequent decrease in the size of the perfusion lesion (39, 40). Furthermore, considering the analogy to MCA ischemia in which it is known that the rate of early reperfusion is on the order of 30% (41), it would seem an unlikely coincidence that all six patients had quick reperfusion. However, whether reperfusion occurred at later time points (slow or prolonged reperfusion) cannot be excluded on the basis of our data.
Could the phenomenon be due to vasogenic edema (eg, occurring during the time point of the second examination)? The mean ADC values at the first and the second examination, respectively, were 5.4 ± 0.4 × 10−4 mm2/s and 5.3 ± 0.5 × 10−4 mm2/s (mean ± SD) (ie, the mean ADC was decreased in comparison to normal tissue at both time points). Furthermore, parts of the ADC lesion at this time point were negative on the T2-weighted images (ie, in four of six patients, the T2 lesion was smaller than the ADC lesion at this time point). This is not a typical finding for vasogenic edema, although it does not entirely exclude some contribution of vasogenic edema.
Another explanation to our observation could be that, for hitherto unknown reasons, an initially small perfusion deficit may induce tissue damage that spreads significantly beyond the initial perfusion lesion. Such a behavior differing from the known lesion evolution in MCA ischemia may be due to a different pathogenesis of small subcortical ischemia (eg, in the AChA territory). Indeed, it is controversial whether the pathogenesis of ischemia in the AChA territory is special. It has been suggested that AChA infarction usually results from small-vessel disease like lacunar strokes and associated carotid artery stenosis, and potential sources of cardiac emboli are rare and may be coincidental (42). Hupperts et al (16) noted that carotid stenosis was more frequent and a cardioembolic source less frequent in AChA infarcts than in other small deep infarcts, but both were less frequent than in superficial infarcts. By contrast, Leys et al (43) concluded that AChA infarcts were rarely related to small-vessel disease and therefore require a complete diagnostic workup. Therefore, no conclusive picture has yet emerged to help explain the difference in lesion evolution we observed.
Another potential explanation of our results may be related to the small lesion size and especially to the relatively poor collateral circulation. It is known that tissue damage caused by cerebral ischemia leads to the release of a number of cytotoxic substances such as free radicals and other inflammatory signals (44). When a large vessel is occluded, the area of tissue damage is surrounded by a large area of less-pronounced perfusion disturbance sustained by collateral circulation. Hence, enlargement of the lesion at the border of the ischemic tissue damage will usually occur within an area of perfusion disturbance. Whether in this case the dominant factor for the lesion growth is the perfusion disturbance or the release of toxic substances at the border of the tissue damage cannot be resolved; perhaps both factors play a role. If, however, the inital perfusion disturbance is confined to a very small area, and the perfusion in the immediate surrounding area is normal for small subcortical infarcts, as our data suggest, then an increase in lesion size may be due to only mechanisms related to cytotoxic events at the border of the tissue damage. It is interesting to note that, in our subjects, the perfusion deficit also increased in size; however, this occurred only after there was already a diffusion lesion (ie, possibly a vicious circle of cytotoxic mechanisms at the lesion border induces secondary perfusion disturbance). In this case, inhibition of such a secondary spread of ischemic damage may be more important than achievement of reperfusion. At present, however, therapeutic consequences remain a matter of speculation. In view of the small number of patients of this initial investigation, further studies with larger sample sizes and improved technology such as diffusion tensor imaging are warranted to address the relevance of this observation.
Conclusion
In this MR imaging study in six patients with acute small subcortical stroke, we found a lesion pattern that differed significantly from the one known for the MCA territory. In MCA infarcts, the initial diffusion lesion typically is smaller than the perfusion deficit and the final infarct develops within the area of the initial perfusion disturbance. In all of our patients with small subcortical infarcts, however, already within 5 hours after stroke onset the diffusion lesion was larger than the perfusion lesion and the final infarct developed into areas outside the initial perfusion deficit. So far, the underlying pathophysiologic mechanisms for these differences in lesion evolution remain a matter of speculation. In view of the small number of patients in our study, further work will be needed to address the relevance of this observation.
Acknowledgments
We thank our colleagues from the clinical research group (Klinische Forschergruppe) for assistance in MR imaging measurements.
Footnotes
Supported by the Deutsche Forschungsgemeinschaft (Klinische Forschergruppe, EI 207/2–3) and by the Bundesministerium für Bildung und Forschung within the competence network “Stroke” (project B5).
Presented in part at the International Society of Magnetic Resonance in Medicine 2001.
C.A.D. and C.M.K. contributed equally to this work.
C.A.D. currently at the Memorial Sloan-Kettering Cancer Center, Department of Biophysics and Biochemistry, New York, NY; C.M.K. currently at F.C. Donders Centre for Cognitive Neuroimaging, Nijmegen, the Netherlands; and B.I.R. currently at Klinik für Psychatrie, Universitäklinikum Charité, Humboldt University, Berlin, Germany.
References
- 1.Le Bihan D, Breton E, Lallemand D, et al. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986;161:401–407 [DOI] [PubMed] [Google Scholar]
- 2.Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497–505 [DOI] [PubMed] [Google Scholar]
- 3.Moseley ME, Cohen Y, Mintorovitch J, et al. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med 1990;14:330–346 [DOI] [PubMed] [Google Scholar]
- 4.Mintorovitch J, Yang GY, Shimizu H, et al. Diffusion-weighted magnetic resonance imaging of acute focal cerebral ischemia: comparison of signal intensity with changes in brain water and Na+, K(+)-ATPase activity. J Cereb Blood Flow Metab 1994;14:332–336 [DOI] [PubMed] [Google Scholar]
- 5.Doege CA, Kerskens CM, Romero BI, et al. MRI of small human stroke shows reversible diffusion changes in subcortical gray matter. Neuroreport 2000;11:2021–2024 [DOI] [PubMed] [Google Scholar]
- 6.Hoehn-Berlage M, Norris DG, Kohno K, et al. Evolution of regional changes in apparent diffusion coefficient during focal ischemia of rat brain: the relationship of quantitative diffusion NMR imaging to reduction in cerebral blood flow and metabolic disturbances. J Cereb Blood Flow Metab 1995;15:1002–1011 [DOI] [PubMed] [Google Scholar]
- 7.Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996;199:391–401 [DOI] [PubMed] [Google Scholar]
- 8.Baird AE, Benfield A, Schlaug G, et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol 1997;41:581–589 [DOI] [PubMed] [Google Scholar]
- 9.Baird AE, Warach S. Magnetic resonance imaging of acute stroke. J Cereb Blood Flow Metab 1998;18:583–609 [DOI] [PubMed] [Google Scholar]
- 10.Barber PA, Darby DG, Desmond PM, et al. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology 1998;51:418–426 [DOI] [PubMed] [Google Scholar]
- 11.Warach S, Wielopolski P, Edelman RR. Identification and characterization of the ischemic penumbra of acute human stroke using echo planar diffusion and perfusion imaging. In: Proc Twelfth Ann Sci Mtg Soc Magn Reson Med 1993;1:263 [Google Scholar]
- 12.Damasio H. A computed tomographic guide to the identification of cerebral vascular territories. Arch Neurol 1983;40:138–142 [DOI] [PubMed] [Google Scholar]
- 13.Donnan GA, Norrving B, Bamford JM, Bogousslavsky J. Subcortical infarction: classification and terminology. Cerebrovasc Dis 1993;3:248–251 [Google Scholar]
- 14.Mohr JP, Steinke W, Timsit SG, et al. The anterior choroidal artery does not supply the corona radiata and lateral ventricular wall. Stroke 1991;22:1502–1507 [DOI] [PubMed] [Google Scholar]
- 15.Helgason CM. A new view of anterior choroidal artery territory infarction. J Neurol 1988;235:387–391 [DOI] [PubMed] [Google Scholar]
- 16.Hupperts RM, Lodder J, Heuts-van RE, Kessels F. Infarcts in the anterior choroidal artery territory: anatomical distribution, clinical syndromes, presumed pathogenesis and early outcome. Brain 1994;117:825–834 [DOI] [PubMed] [Google Scholar]
- 17.Herman LH, Fernando OU, Gurdjian ES. The anterior choroidal artery: an anatomical study of its area of distribution. Anat Rec 1966;154:95–102 [DOI] [PubMed] [Google Scholar]
- 18.Meier P, Zierler KL. On the theory of the indicator-dilution method for measurement of blood flow and volume. J Appl Physiol 1954;6:731–744 [DOI] [PubMed] [Google Scholar]
- 19.Belliveau JW, Rosen BR, Kantor HL, et al. Functional cerebral imaging by susceptibility-contrast NMR. Magn Reson Med 1990;14:538–546 [DOI] [PubMed] [Google Scholar]
- 20.Rosen BR, Belliveau JW, Aronen HJ, et al. Susceptibility contrast imaging of cerebral blood volume: human experience. Magn Reson Med 1991;22:293–299 [DOI] [PubMed] [Google Scholar]
- 21.Heiss WD, Kracht LW, Thiel A, et al. Penumbral probability thresholds of cortical flumazenil binding and blood flow predicting tissue outcome in patients with cerebral ischaemia. Brain 2001;124:20–29 [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa Y, Fisher M, Latour LL, et al. MRI diffusion mapping of reversible and irreversible ischemic injury in focal brain ischemia. Neurology 1994;44:1484–1490 [DOI] [PubMed] [Google Scholar]
- 23.Hoehn-Berlage M, Norris DG, Kohno K, et al. Early infarct evolution in rat brain: NMR diffusion imaging, regional blood flow, ATP and tissue pH. In: The Proceedings of the 12th Annual Meeting of the Society of Magnetic Resonance Imaging in Medicine.1993;1:250 [Google Scholar]
- 24.Jiang Q, Zhang ZG, Chopp M, et al. Temporal evolution and spatial distribution of the diffusion constant of water in rat brain after transient middle cerebral artery occlusion. J Neurol Sci 1993;120:123–130 [DOI] [PubMed] [Google Scholar]
- 25.Mintorovitch J, Moseley ME, Chileuitt L, et al. Comparison of diffusion- and T2-weighted MRI for the early detection of cerebral ischemia and reperfusion in rats. Magn Reson Med 1991;18:39–50 [DOI] [PubMed] [Google Scholar]
- 26.Weber J, Mattle HP, Heid O, et al. Diffusion-weighted imaging in ischaemic stroke: a follow-up study. Neuroradiology 2000;42:184–191 [DOI] [PubMed] [Google Scholar]
- 27.Uluğ AM, Beauchamp NJ, Bryan RN, van Zijl PC. Absolute quantitation of diffusion constants in human stroke. Stroke 1997;28:483–90 [DOI] [PubMed] [Google Scholar]
- 28.Karonen JO, Liu Y, Vanninen RL, et al. Combined perfusion- and diffusion-weighted MR imaging in acute ischemic stroke during the 1st week: a longitudinal study. Radiology 2000;217:886–894 [DOI] [PubMed] [Google Scholar]
- 29.Parsons MW, Yang Q, Barber PA, et al. Perfusion magnetic resonance imaging maps in hyperacute stroke. Stroke 2001;32:1581–1587 [DOI] [PubMed] [Google Scholar]
- 30.Vonken EJPhA, van Osch MJP, Bakker CJG, Viergever MA. Measurement of cerebral perfusion with dual-echo multi-slice quantitative dynamic susceptibility contrast MRI. J Magn Reson Imaging 1999;10:109–117 [DOI] [PubMed] [Google Scholar]
- 31.Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization: normal values and effect of age. Brain 1990;113:27–47 [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa T, Kawamura S, Hadeishi H, et al. Cerebral blood flow and oxygen metabolism in hemiparetic patients with chronic subdural hematoma: quantitative evaluation using positron emission tomography. Surg Neurol 1995;43:130–136 [DOI] [PubMed] [Google Scholar]
- 33.Pantano P, Baron JC, Lebrun-Grandie P, et al. Regional cerebral blood flow and oxygen consumption in human aging. Stroke 1984;15:635–641 [DOI] [PubMed] [Google Scholar]
- 34.Rempp KA, Brix G, Wenz F, et al. Quantification of regional cerebral blood flow and volume with dynamic susceptibility contrast-enhanced MR imaging. Radiology 1994;193:637–641 [DOI] [PubMed] [Google Scholar]
- 35.Iida H, Akutsu T, Endo K, et al. A multicenter validation of regional cerebral blood flow quantitation using [123I]iodoamphetamine and single photon emission computed tomography. J Cereb Blood Flow Metab 1996;16:781–793 [DOI] [PubMed] [Google Scholar]
- 36.Mayer TE, Hamann GF, Baranczyk J, et al. Dynamic CT perfusion imaging of acute stroke. AJNR Am J Neuroradiol 2000;21:1441–1449 [PMC free article] [PubMed] [Google Scholar]
- 37.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J 1994;66:259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark CA, Hedehus M, Moseley ME. In vivo mapping of the fast and slow diffusion tensors in human brain. Magn Reson Med 2002;47:623–628 [DOI] [PubMed] [Google Scholar]
- 39.Kidwell CS, Saver JL, Mattiello J, et al. Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol 2000;47:462–469 [PubMed] [Google Scholar]
- 40.Parsons MW, Barber PA, Chalk J, et al. Diffusion- and perfusion-weighted MRI response to thrombolysis in stroke. Ann Neurol 2002;51:28–37 [DOI] [PubMed] [Google Scholar]
- 41.Marchal G, Furlan M, Beaudouin V, et al. Early spontaneous hyperperfusion after stroke: a marker of favourable tissue outcome? Brain 1996;119:409–419 [DOI] [PubMed] [Google Scholar]
- 42.Bruno A, Graff-Radford NR, Biller J, Adams HPJ. Anterior choroidal artery territory infarction: a small vessel disease. Stroke 1989;20:616–619 [DOI] [PubMed] [Google Scholar]
- 43.Leys D, Mounier-Vehier F, Lavenu I, et al. Anterior choroidal artery territory infarcts: study of presumed mechanisms. Stroke 1994;25:837–842 [DOI] [PubMed] [Google Scholar]
- 44.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999;22:391–397 [DOI] [PubMed] [Google Scholar]