Abstract
Summary: Anomalies of the course of the facial nerve have been reported in association with middle and inner ear malformations. Bifurcation of its intratemporal portion is a rare malformation in which focal splitting of one or more facial nerve segments occurs. We describe the CT appearance of this anomaly and discuss its possible embryology. Facial nerve bifurcation is important to recognize in patients undergoing evaluation for congenital hearing loss and other congenital ear malformations.
The exquisite detail afforded by a dedicated CT scan of the temporal bone allows evaluation of osseous abnormalities that may have previously gone unnoticed. We describe three cases of bifurcation of the facial nerve (CN7), a rare congenital malformation that is associated with dysplasia of the middle and inner ear. Facial nerve bifurcation, although well described in the surgical literature, has not been reported in the radiology literature.
The three cases presented herein were of patients referred for temporal bone CT as part of an evaluation of hearing loss. None of these patients had been previously suspected of having a congenital facial nerve malformation. In all cases, CT scans were obtained as direct axial and coronal 1-mm sections through the temporal bones, processed with a high spatial frequency (bone) algorithm, and viewed with wide windows.
Case Reports
Case 1
A 6-year-old girl was evaluated for hearing loss that had been detected at school screening. She had a history of otitis media that had been treated previously with bilateral tympanostomy tubes without significant improvement. On clinical examination, there was bilateral mixed hearing loss, and no facial nerve abnormalities were noted. Audiometry revealed bilateral low-frequency conductive hearing loss up to 50 dB. On serologic evaluation, she was noted to have an elevated erythrocyte sedimentation rate (ESR, 51), slightly elevated antinuclear antibodies (ANA) (1:80), but negative rheumatoid factor, as well as a positive anti 68kD antibody, which is seen in approximately 70% of patients with autoimmune hearing loss.
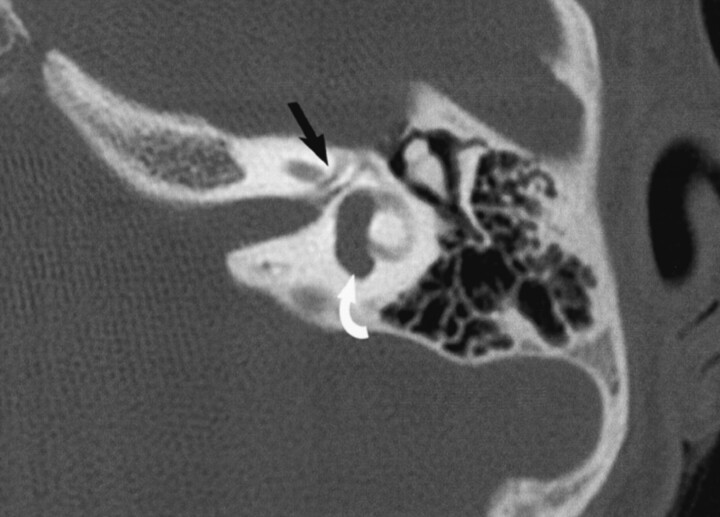
A temporal bone CT scan demonstrated a dilated left posterior semicircular canal; the remainder of the bony labyrinthine structures appeared normal. There was a normal internal auditory canal on the left; however, two labyrinthine facial nerve canals of identical size were noted (Fig 1). The tympanic and mastoid segments of the left facial nerve appeared normal, and no middle ear malformations were evident. The right temporal bone and facial nerve canal also appeared normal. High-spatial-resolution axial 3D fast spin-echo MR imaging was also performed, confirming the posterior semicircular canal malformation, but the dual labyrinthine CN7 canals were very difficult to appreciate.
Fig 1.
Case 1, 6-year-old girl evaluated for mixed hearing loss, worse on the left side. Axial 1-mm section at the superior aspect of the left internal auditory canal (IAC) showns two canals coursing anteriorly in the expected location of the labyrinthine segment of the facial nerve (arrow). Note also the dilated posterior semicircular canal (curved arrow).
Case 2
A 12-year-old boy presented with a 10-year history of bilateral hearing loss. Right-sided facial nerve palsy had been present since birth and was attributed to a traumatic forceps delivery. There was a history of right ear surgery (of unknown nature) in Mexico at the age of 8 years, and from the family’s account this had not altered hearing or facial nerve function. On examination, the external auditory canals were bilaterally stenotic, and the right hemimandible hypoplastic. The clinical and audiometric examinations were consistent with bilateral conductive hearing loss, which was worse on the right. A right-sided facial nerve palsy was evident (5/6 House-Brackmann grade) and the left facial nerve function was normal (1/6).
A CT scan demonstrated subtle dysplasia of the stapes bilaterally and an atresia of the right oval window. The contour, course, and position of the left facial nerve were normal.
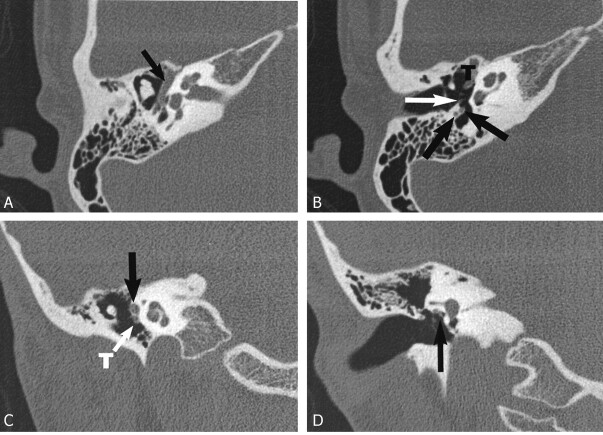
On the right side, the labyrinthine portion of CN7 appeared normal, although the proximal tympanic segment was asymmetrically thickened. Two tympanic segments of CN7 were clearly identified in the coronal plane, one coursing in the normal position of the CN7 canal and the second coursing over a stenotic oval window. Two nerves were identified in the facial recess, and two canals within the mastoid coursed inferiorly into two separate stylomastoid foramina (Fig 2).
Fig 2.
Case 2, 12-year-old boy with right-side mixed HL. Axial 1-mm CT sections through the level of the cochlear modiolus and the malleo-incudal joint (A) demonstrate a thickened tympanic CN7 (black arrow). The more inferior axial section at the level of the round window (B) shows two separate proximal mastoid segment nerves. The stapedius tendon is distinctly seen separate from these nerve elements (white arrow). The coronal images confirm a thickened proximal tympanic nerve (C, black arrow) and two segments more posteriorly at the level of the oval window (D). The more inferior of the bifid segments courses lateral to a stenotic oval window (arrow). T = tensor tympani tendon.
Case 3
A 40-year-old woman with congenital sensorineural hearing loss was evaluated for possible cochlear implantation. There was a history of premature birth and subsequent developmental delay with poor mental function as an adult. Right-side facial weakness had been noted at birth. She had prior bilateral branchial cleft cyst excisions, and there was a more recent history of severe trauma with a basal skull fracture. On examination in the clinic, weakness of the right marginal mandibular branch of CN7 was noted. The clinical impression was of branchio-oto-renal syndrome with bilateral profound hearing loss, which audiometry showed to be of a mixed nature.
CT demonstrated severe bilateral cochleo-vestibular dysplasia with bilateral oval window stenosis. In addition, there were bilateral middle ear malformations with dysplastic ossicular masses annealed to the lateral wall of the middle ear cavity.
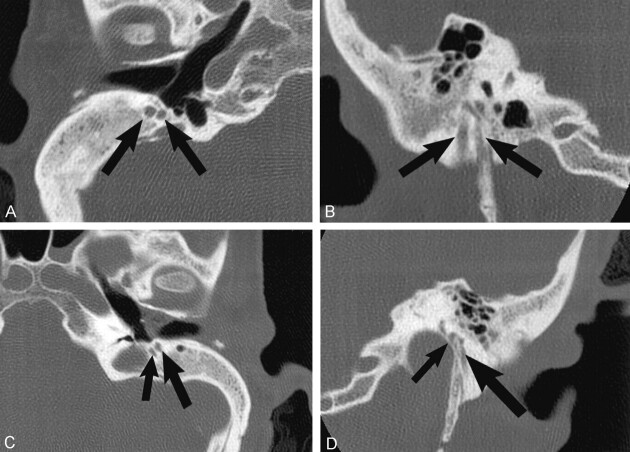
Each facial nerve had a bony canal separate from the internal auditory canal, passing anteriorly and superiorly in a looplike fashion from the cerebellopontine angle to the geniculate ganglion. The tympanic segments of the facial nerve had a normal course through the middle ear cavities on each side. On the right side the proximal mastoid appeared symmetrically bifid, with two mastoid segments and separate stylomastoid foramina.
Mastoid segment CN7 bifurcation was also present on the left side, where there was a small medial branch that exited the skull base through the lateral wall of the jugular foramen (Fig 3).
Fig 3.
Case 3, 40-year-old woman with branchio-oto-renal syndrome and bilateral congenital HL. Axial (A) and coronal (B) images of the right side demonstrate two distinct symmetric mastoid segments of CN7 (arrows) that exit via separate stylomastoid foramina. On the left side, bifid mastoid CN7 segments are also seen (arrows, axial image C), with the smaller, medial segment exiting into the lateral wall of the jugular foramen (smaller arrow, coronal image D). Additional axial (E) image of the labyrinthine portion of CN7 on the left side shows a looplike anterior course separate to the internal auditory canal (arrow). This anomalous CN7 course was seen bilaterally, and there was bilateral severe cochleo-vestibular dysplasia.
Discussion
Many abnormalities of the facial nerve canal in the petrous temporal bone have been documented. The most common is congenital bony dehiscence of the CN7 canal that occurs in up to 55% of otherwise normal temporal bones, predominantly involving the tympanic portion (91%) (1). With this high prevalence, it is more accurately described as a variation in normal anatomy (2, 3). Anomalies of the CN7 canal are infrequently found in normal temporal bones and are usually seen in association with middle and inner ear dysplasias (3–8). Anteromedial displacement of the labyrinthine segment of CN7 has been described in association with cochlear malformations, and an anteriorly displaced CN7 mastoid segment is often noted with congenital aural atresia (9, 10). An anomalous course of the tympanic segment has also been reported in association with oval window atresia (11, 12).
Bifurcation and trifurcation anomalies of CN7 have been previously described in the otolaryngology literature. These have been reported to involve all portions of the nerve from the intracanalicular segment to the mastoid segment, with the most common anomalies occurring along the tympanic segment (3, 13–16). Bifurcation of the intracanalicular CN7 has been reported both within one canal and with a double internal auditory canal containing a facial nerve in each. In both case reports, there was a strong facial response to stimulation of each facial nerve segment (13, 14).
Bifurcation of the labyrinthine CN7 segment, as with case 1, appears to be the most rare of the intratemporal anomalies. We cannot find any English-language reports of similar labyrinthine bifurcations, although Nager cites two German articles, from 1933 and 1963 (3). Although the dual labyrinthine canals possibly represented one for the facial nerve and one for the nervus intermedius, the latter nerve is significantly smaller than the facial nerve, yet the canals were of symmetric diameter.
Prior reports of bifurcation of the tympanic CN7 segment predominantly described focal bifurcation of the nerve proximal or anterior to the oval window with rejoining of the segments distal to the stapes or at the posterior genu. At surgery, stimulation of both branches has been reported to produce a strong facial response (15). A bifid tympanic facial nerve has also been described where one segment passed through the stapedial arch (4). Tympanic segment anomalies are associated with developmental anomalies of the vestibular fenestra and stapes as with case two. In case 2, however, the bifid CN7 segments did not rejoin within the temporal bone but exited through separate stylomastoid foramina. A prior report showed the CT findings in a case of tympanic CN7 bifurcation that was not detected preoperatively, although it did not describe the full extent of the bifid segments (15).
Bifurcation of CN7 posterior or distal to the oval window usually results in two mastoid segments in separate bony canals, with separate stylomastoid foramina. The lateral segment is reported to be the larger and receives the chorda tympani nerve (3, 5). In case 3, the lateral segment of the bifid mastoid CN7 was indeed the larger of the two. The right-side mastoid CN7, however, had almost symmetric bifid segments within separate stylomastoid foramina. Bifid mastoid segments have been described as crossing within the temporal bone or uniting at or just outside the foramen. Trifurcation of the CN7 mastoid segment with separate bony canals and foramina has also been demonstrated surgically (3, 6).
The embryologic origin of facial nerve bifurcation anomalies is uncertain, because CN7 never exists as separate bundles during its development. Formation of the nerve commences early in gestation with the facioacoustic primordium (derived from the neural crest and otic vesicle) separating into facial and acoustic components at the end of the 4th week. By the end of the 5th gestational week, the chorda tympani has differentiated from the distal facial nerve. By the 8th week, the orientation of CN7 within the temporal bone has been established, with the nerve’s ultimate position and bony covering determined by development of the stapes and membranous labyrinth.
The basic configuration of the facial nerve is completed at around 8 weeks, whereas the mesenchymal tissue through which it passes and the structures it innervates are still poorly formed.
Abnormal separation of CN7 much earlier in embryogenesis may be necessary for a bifurcation or trifurcation anomaly to result. Early division of the nerve at around 4–6 weeks may allow the displacement of one segment by the developing temporal bone structures, such as pulling it anteriorly or laterally (8, 13, 14). An early global insult to the fetus may also disrupt stapedial or labyrinthine formation, resulting in their dysplasia. This association of facial nerve abnormalities and middle and inner ear dysplasia has already been established and is supported by the cases presented.
Facial canal ossification commences near the end of the 5th month in utero from second branchial arch cartilage (Reichert’s cartilage), but the mastoid segment canal largely forms postnatally with growth of the mastoid bone (3, 5, 8, 17–19).
Although the cases presented did not have surgical or pathologic confirmation, the images are highly suggestive of the bifurcation anomalies described in the surgical literature. The appearance in case 2 was thought to be so convincing for a bifid segment overlying the oval window that the contralateral ear was selected for stapedectomy and piston placement. The presence of an anomalous CN7 component over the window may limit or preclude surgical access for stapedectomy, and an anomalous CN7 segment through the mastoid bone may be at risk with mastoidectomy or cochlear implant placement (16).
Conclusion
Because of the association of facial nerve anomalies with middle and inner ear malformations, the facial nerve should be carefully evaluated on all temporal bone CT scans of patients with congenital hearing loss and other congenital ear malformations. Although bifurcation of the facial nerve appears to be rare, its detection is important in the presurgical evaluation of patients to avoid potential complications as well as to direct the radiologist to look for the presence of other congenital anomalies. The use of dedicated high-spatial-resolution CT maximizes conspicuity of temporal bone anatomy, and a heightened awareness of anomalies of the facial nerve and its course will aid in its radiologic detection.
References
- 1.Baxter A. Dehiscence of the fallopian canal. J Laryngol Otol 1971;85:587–594 [DOI] [PubMed] [Google Scholar]
- 2.Jahrsdoerfer RA. The facial nerve in congenital middle ear malformations. Laryngoscope 1981;91:1217–1225 [DOI] [PubMed] [Google Scholar]
- 3.Nager GT. Pathology of the Ear and Temporal Bone. Philadelphia: Lippincott Williams & Wilkins;1993;147–164
- 4.Marquet J. Congenital malformations and middle ear surgery. J R Soc Med 1981;74:119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basek M. Anomalies of the facial nerve in normal temporal bones. Ann Otol Rhinol Laryngol 1962;71:382–390 [DOI] [PubMed] [Google Scholar]
- 6.Fowler EP. Variations in the temporal bone course of the facial nerve. Laryngoscope 1961;71:937–944 [DOI] [PubMed] [Google Scholar]
- 7.Nager GT, Proctor B. The facial canal: normal anatomy, variations and anomalies. II. Anatomical variations and anomalies involving the facial canal. Ann Otol Rhinol Laryngol Suppl 1982;97:45–61 [PubMed] [Google Scholar]
- 8.Durcan DJ, Shea JJ, Sleeckx JP. Bifurcation of the facial nerve. Arch Otolaryngol 1967;86:619–631 [DOI] [PubMed] [Google Scholar]
- 9.Romo LV, Curtin HD. Anomalous facial nerve canal with cochlear malformations. AJNR Am J Neuroradiol 2001;22:838–844 [PMC free article] [PubMed] [Google Scholar]
- 10.Remley KB, Swartz JD, Harnsberger HR. The external auditory canal. In: Swartz JD, Harnsberger HR, eds. Imaging of theTemporal Bone. 3rd ed. New York: Thieme1997. :16–46
- 11.Zeifer B, Sabini P, Sonne J. Congenital absence of the oval window: radiologic diagnosis and associated anomalies. AJNR Am J Neuroradiol 2000;21:322–327 [PMC free article] [PubMed] [Google Scholar]
- 12.Booth TN, Vezina LG, Karcher G, Dubovsky EC. Imaging and clinical evaluation of isolated atresia of the oval window. AJNR Am J Neuroradiol 2000;21:171–174 [PMC free article] [PubMed] [Google Scholar]
- 13.Celin SE, Wilberger JE, Chen DA. Facial nerve bifurcation within the internal auditory canal. Otolaryngol Head Neck Surg 1991;104:389–393 [DOI] [PubMed] [Google Scholar]
- 14.Curtin H, May M. Double internal auditory canal associated with progressive facial weakness. Am J Otol 1986;7:275–281 [PubMed] [Google Scholar]
- 15.Kieff DA, Curtin HD, Healy GB, Poe DS. A duplicated tympanic facial nerve and congenital stapes fixation: an intraoperative and radiographic correlation. Am J Otolaryngol 1998;19:283–286 [DOI] [PubMed] [Google Scholar]
- 16.Raine CH, Hussain SS, Khan S, Setia RN. Anomaly of the facial nerve and cochlear implantation. Ann Otol Rhinol Laryngol Suppl 1995;166:430–431 [PubMed] [Google Scholar]
- 17.Jahrsdoerfer RA. Embryology of the facial nerve. Am J Otol 1988;9:423–426 [PubMed] [Google Scholar]
- 18.Sperber GH. Craniofacial Embryology. 4th ed. Oxford: Wright;1989. :49 , 62–64
- 19.Sataloff RT. Embryology of the facial nerve and its clinical applications. Laryngoscope 1990;100:969–984 [DOI] [PubMed] [Google Scholar]