Abstract
Summary: We present a case of increased signal intensity of the cerebrum (symmetric involvement of the paraventricular thalamus and external capsule) and cerebellum on both T1- and T2-weighted images in a patient with documented heat stroke. An ischemic and hemorrhagic mechanism is proposed, and the contributions of the direct effects of hyperthermia are discussed.
Heat stroke, whether classic or exertional, is a medical emergency defined by a body temperature greater than 40°C (104°F) that causes altered mental status and deterioration of multiple organ systems (1, 2). The central nervous system is very sensitive to hyperthermia, causing neurologic complications due to involvement of the cerebellum, basal ganglia, anterior horn cells, and peripheral nerves (2, 3). CT and MR imaging studies have shown loss of gray matter–white matter discrimination, white matter involvement, and cerebellar atrophy (2, 3).
Case Report
A 17-year-old male patient was brought to the medical center with symptoms of confusion and combativeness after doing heavy outdoor exercise in July. His body temperature was recorded to be 106.8°F. The day prior, the patient had experienced malaise, nausea, vomiting, and mild diarrhea. The patient was initially intubated and treated with external ice packs and gastric ice lavage, reducing his temperature to 105°F after 2.5 hours and 98.8°F after 5 hours. Within the first week after admission, the patient experienced disseminated intravascular coagulation, continued intestinal dysfunction, rhabdomyolysis, elevated liver function, acute renal insufficiency, and urosepsis. These complications resolved, but 1 week after admission the patient was noted to have persistent mental status changes and memory deficits. The patient also had difficulty with fine motor coordination, ataxia with shuffling gait, and problems with balance and coordination, including dysmetria on finger-to-nose testing, which became more apparent as strength improved.
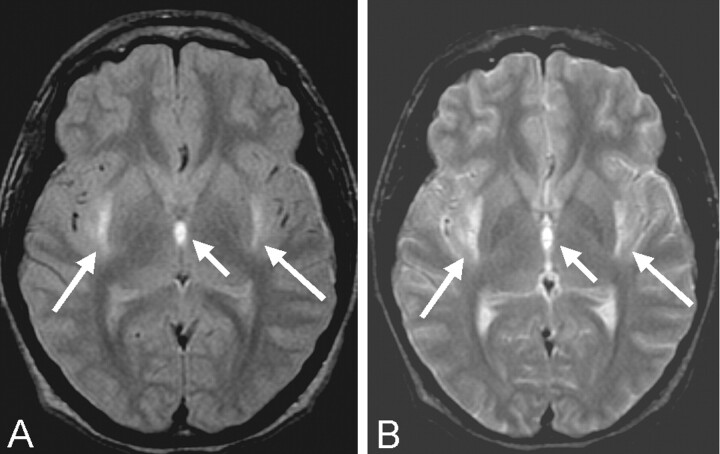
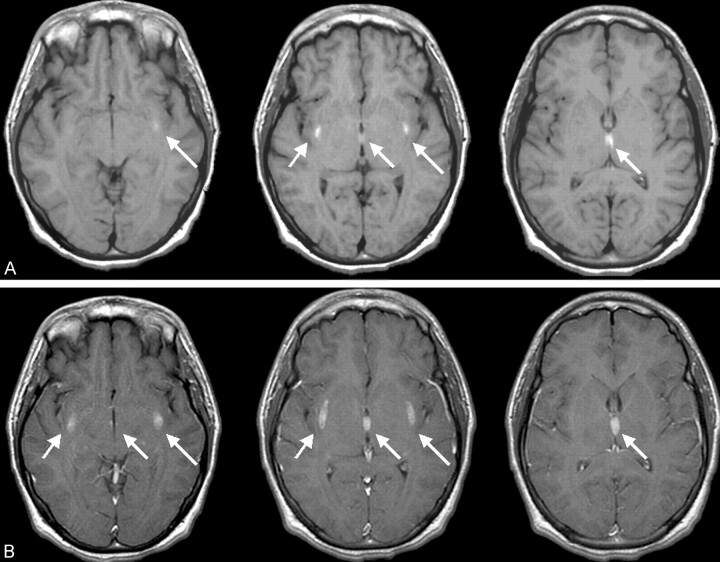
MR imaging of the brain was therefore performed. Proton density–and T2-weighted images revealed hyperintensity of the external capsules and adjacent lateral putamen, the paraventricular nucleus of the thalamus, and scattered areas throughout the cerebellar hemispheres (Fig 1). Pre- and postcontrast T1-weighted images demonstrated T1 shortening and accompanying enhancement only in the supratentorial lesions (Fig 2). T1 shortening in the paraventricular nucleus of the thalamus bordering the third ventricle also extended into the third ventricle, suggesting an underlying hemorrhagic cause, resulting from extracellular methemaglobin (Fig 2). Areas of T2-weighted changes in the cerebellum had corresponding darkness on T1-weighted images but were also noted to enhance after contrast medium administration (Fig 3). These findings suggest an ischemic and hemorrhagic small vessel process, both supra- and infratentorial.
Fig 1.
Proton density–(A) and T2-weighted (B) axial MR images of the brain at the level of the third ventricle show hyperintensity (arrows) in the external capsules and medial thalami and altered signal intensity in the adjacent third ventricle.
Fig 2.
Three axial precontrast contiguous T1-weighted images (A) and corresponding postcontrast images (B) demonstrate both T1 shortening and accompanying enhancement in the same area (arrows).
Fig 3.
Axial postcontrast T1-weighted image shows scattered cerebellar hyperintensity and patchy enhancement in the cerebellar hemispheres bilaterally (arrows).
Discussion
Heat stroke can cause many different reactions within the body that lead to neurologic dysfunction, including decreased cerebral perfusion and aberrations in coagulation. Initially during hyperthermia, peripheral vasodilatation predominates to facilitate heat loss through the skin. To avoid a functional hypovolemia, a compensatory vasoconstriction of the splanchnic and renal vasculature occurs, likely causing the symptoms of nausea, vomiting, and diarrhea. If heat stress continues, however, the compensatory vasoconstriction will eventually fail, further increasing the body temperature. Concurrently, cerebrovascular congestion and cerebral edema occur with the hyperthermia, causing an increase in intracranial pressure. Combined with a failure of splanchnic vasoconstriction and decreased mean arterial pressure, cerebral blood flow falls (4, 5). This results in cerebral ischemia. An aberration in coagulation is caused by a decrease in protein C, protein S, and antithrombin III, as well as alterations in vascular endothelium, creating a pattern resembling sepsis and disseminated intravascular coagulation (4). This can cause hemorrhage within the brain, also resulting in neurologic dysfunction (5). All of these physiologic processes occur concurrently, causing the common outcome of neuronal dysfunction.
It is of interest that, in the cerebellum, thermal injury itself may cause destruction of the Purkinje cells. Increased production of heat shock proteins within the rabbit cerebellum during hyperthermia indicates that Purkinje cells may have an increased demand for thermal injury repair. Therefore, in addition to being susceptible to hypoxic-ischemic injury, there is evidence that Purkinje cells are also susceptible to direct thermal injury (6).
Although the pathophysiologic mechanism of neuronal damage is fairly well understood and known to occur in heat stroke, documentation of these changes on MR images is infrequent. Most reports of MR imaging findings have revealed delayed cerebellar atrophy in these patients. Biary et al (7) described a patient who developed such cerebellar atrophy, while also noting patchy areas of white matter hyperintensity in both cerebral hemispheres and in the left striatum. Other reported cerebral MR imaging findings include abnormal signal intensity in the pons in a case of central pontine myelinolysis in a patient with heat stroke (8). High signal intensity on T2-weighted images of the periventricular frontal lobe white matter were noted in a 9-month-old patient with congenital sensory neuropathy and anhidrosis after an episode resembling heat stroke. Fourteen months later, MR imaging revealed atrophic changes that were consistent with delayed myelination (9). Mild, diffuse atrophy was also seen at MR imaging performed in a 12-year-old patient 11.5 months after developing Kluver-Bucy syndrome after the development of heat stroke (10). In another case, a 10-month-old patient demonstrated vascular boundary zone infarcts at MR imaging 40 days after the development of heat stroke (11).
The MR imaging findings in the present case demonstrate a unique symmetric enhancement, T1 shortening, and T2 prolongation in the external capsules bordering the lateral putamen as well as the thalamus immediately bordering the third ventricle. These cerebral lesions could represent a deep brain manifestation of failure of autoregulation similar to the lesions seen in the 10-month-old patient reported by Akaboshi and Miyashita (11). A pattern of T2 prolongation specifically seen in the external capsules has been reported to be characteristic of, although not specific for, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) as well as more sporadically in patients with hypertension. This pattern is consistent with a small vessel process caused by ischemia, demyelination, and axonal degeneration resulting in abnormal T2-weighted signal intensity (12). Specifically in CADASIL patients, these white matter areas of high signal intensity can progress to subcortical lacunar lesions at the gray matter–white matter junction. Subcortical lacunar lesions have the signal intensity of CSF because of leakage of CSF into the widened perivascular space of the small vessels this disease affects (13). To our knowledge, however, the present case reveals T1 shortening as well as T2 prolongation, a unique and distinct supratentorial manifestation likely reflecting the ischemic and hemorrhagic intracranial effect of heat stroke.
Although heat can affect all organ systems adversely, it is known that the cerebellum is especially susceptible (1). The Purkinje cells within the cerebellum are, in particular, vulnerable to thermal injury. As noted above, it has been suggested the hyperthermia itself, as opposed to the resultant hypoxic or ischemic insults, causes the destruction of Purkinje cells (1, 6). This neuronal destruction then causes generalized cerebellar atrophy, which has been widely reported as evidenced by MR imaging (1, 3, 7); however, to our knowledge no reports of bilateral signal intensity alterations and enhancement of the cerebellum exist in the literature. This suggests that, in addition to the likely ischemic and hemorrhagic small vessel process seen supratentorially, we are also seeing it infratentorially. Hyperintensity on both T1- and T2-weighted images of the cerebrospinal spaces of the third ventricle also suggests an ischemic and hemorrhagic mechanism, implying spread of intracellular methemoglobin into the third ventricle from adjacent hemorrhagic infarction (Figs 1 and 2).
The present case demonstrates a unique new finding of symmetric hyperintensity in the external capsules and thalami, an unusual pattern not previously described in the setting of heat stroke. The presence of specific subacute enhancing T2-weighted signal intensity abnormalities in the cerebellum has also not been previously described and is likely the precursor of the reported long-term sequelae of cerebellar atrophy. The enhancement reflects a breakdown of the blood-brain barrier. As stated above, the physiology of heat stroke includes increased intracranial pressure combined with autonomic dysfunction that lead to cerebral hypoperfusion and ischemia and a tendency toward intracranial hemorrhage because of abnormal coagulation. We propose that these known physiologic effects of heat stroke are the underlying causes for the supratentorial findings. The cerebellar findings may be a combination of these factors coupled with or solely due to the directly destructive effects of hyperthermia on the Purkinje cells.
Clinically, the patient’s memory deficits and mental status change are likely due to the damaging effects to the external capsule, which is known to be a source of input to the amygdala and has been associated with emotional memory (14).
The differential diagnosis for bilateral high signal intensity in the external capsule and lateral putamen on T2-weighted images includes not only CADASIL and hypertension, but also methanol toxicity and, rarely, Wilson disease (15, 16). The symmetric T1 shortening in this location, however, is unique to this case and again likely reflects a very specific hemorrhagic consequence of an underlying ischemic process uniquely affecting these areas as well as those in the thalamus immediately adjacent to the third ventricle. Such T1 shortening has been described in neurofibromatosis and liver failure but typically involves more medial structures such as the globus pallidus and thalamus (17, 18). Although the T1 shortening could alternatively relate to the patient’s transient liver dysfunction, the pattern of spread into the third ventricle and exclusively extreme lateral and medial deep gray matter involvement are not typical of this or any of these previously described metabolic (nonhemorrhagic) scenarios of T1 shortening.
The symmetric medial thalamic lesions correspond to the paraventricular nucleus. This is known to be intimately associated with the systems that regulate core temperature, metabolism, and energy balance via the hypothalamic-pituitary-adrenal axis (19). It is therefore likely that abnormal signal intensity in this structure relates to the excess demands placed upon it in the setting of this patient’s acute hyperthermia. Such increased local metabolic demands could therefore have outstripped the capabilities of the blood supply to this region, compounded by impaired cerebral perfusion, resulting in ischemic, hemorrhagic effect.
Conclusion
This case demonstrates ischemic and hemorrhagic side effects of heat stroke in both the cerebral hemispheres and the cerebellum. To our knowledge, this is the first case to report involvement of the external capsules and thalamus, which exhibited symmetric T1 shortening, T2 prolongation, postcontrast enhancement, and an enhancing phase in the more common sites of cerebellar injury.
Footnotes
The opinions or assertions contained herein are the private views of the authors and are not to be construed as reflecting the views of the Department of the Army or the Department of Defense.
References
- 1.Albukrek D, Bakon M, Moran DS, et al. Heat-stroke-induced cerebellar atrophy: clinical course, CT and MRI findings. Neuroradiology 1997;39:195–197 [DOI] [PubMed] [Google Scholar]
- 2.Szold O, Reider-Groswasser II, Abraham RB, et al. Gray-white matter discrimination: a possible marker for brain damage in heat stroke? Eur J Radiol 2002;43:1–5 [DOI] [PubMed] [Google Scholar]
- 3.Kalita J, Misra UK. Neurophysiological studies in a patient with heat stroke. J Neurol 2001;248:993–995 [DOI] [PubMed] [Google Scholar]
- 4.Bouchama A, Knochel JP. Medical progress: heat stroke. N Engl J Med 2002;346:1978–1988 [DOI] [PubMed] [Google Scholar]
- 5.Yarbrough B, Vicario S. Heat illness. In: Marx J, ed. Rosen’s Emergency Medicine. Concepts and Clinical Practice. 5th ed. St Louis, Mo: Mosby;2002. :1997–2009
- 6.Van Stavern GP, Biousse V, Newman NJ, Leingang JC. Downbeat nystagmus from heat stroke. J Neurol Neurosurg Psychiatry 2000;69:403–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biary N, Madkour MM, Sharif H. Post-heatstroke parkinsonism and cerebellar dysfunction. Clinical Neurol Neurosurg 1995;97:55–57 [DOI] [PubMed] [Google Scholar]
- 8.McNamee T, Forsythe S, Wollmann R, Ndukwu IM. Central pontine myelinolysis in a patient with classic heat stroke. Arch Neurol 1997;54:935–936 [DOI] [PubMed] [Google Scholar]
- 9.Iwanaga R, Matsuishi T, Ohnishi A, et al. Serial magnetic resonance images in a patient with congenital sensory neuropathy with anhidrosis and complications resembling heat stroke. J Neurol Sci 1996;142:79–84 [DOI] [PubMed] [Google Scholar]
- 10.Pitt DC, Kriel RL, Wagner NC, Krach LE. Kluver-Bucy syndrome following heat stroke in a 12-year-old girl. Pediatric Neurol 1995;13:73–76 [DOI] [PubMed] [Google Scholar]
- 11.Akaboshi S, Miyashita A. A case of heat stroke with cortical laminar necrosis on vascular boundary zones. No To Hattatsu 1996;28:434–437 [PubMed] [Google Scholar]
- 12.O’Sullivan M, Jarosz JM, Martin RJ, et al. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology 2001;56:628–634 [DOI] [PubMed] [Google Scholar]
- 13.Van Den Boom R, Lesnik Oberstein SA, Van Duinen SG, et al. Subcortical lacunar lesions: an MR imaging finding in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Radiology 2002;224:791–796 [DOI] [PubMed] [Google Scholar]
- 14.Aroniadou-Anderjaska V, Post RM, Rogawski MA, Li H. Input-specific LTP and depotentiation in the basolateral amygdala. Neuroreport 2001;12:635–640 [DOI] [PubMed] [Google Scholar]
- 15.Feany MB, Anthony DC, Frosch MP, et al. Two cases with necrosis and hemorrhage in the putamen and white matter. Brain Pathol 2001;11:121–125 [PubMed] [Google Scholar]
- 16.Amato C, Bisceglie P, Moschini M. Cerebral magnetic resonance in Wilson’s disease. Radiol Med (Torino) 1994;88:752–757 [PubMed] [Google Scholar]
- 17.Terada H, Barkovich AJ, Edwards MS, Ciricillo SM. Evolution of high-intensity basal ganglia lesions on T1-weighted MR in neurofibromatosis type 1. AJNR Am J Neuroradiol 1996;17:755–760 [PMC free article] [PubMed] [Google Scholar]
- 18.Vymazal J, Babis M, Brooks RA, et al. T1 and T2 alterations in the brains of patients with hepatic cirrhosis. AJNR Am J Neuroradiol 1996;17:333–336 [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatnagar S, Dallman MF. The paraventricular nucleus of the thalamus alters rhythms in core temperature and energy balance in a state-dependent manner. Brain Res 1999;851:66–75 [DOI] [PubMed] [Google Scholar]