Abstract
Summary: We report the initial and follow-up brain findings in a 42-year-old male patient with CNS involvement with African trypansomiasis. Initial MR imaging demonstrated diffuse hyperintensity in the basal ganglia bilaterally as well as involvement of the internal capsule, external capsule, and extreme capsule. Follow-up examination at 1 year revealed decreased signal intensity in the previously affected areas; however, ventricular enlargement indicative of atrophy was readily apparent.
African trypanosomiasis (AT) is a disease caused by Trypanozoon brucei. This disease can occur with or without central nervous system involvement. The prognosis of the latter is poor (1). Because this disease progresses quickly and can result in permanent brain damage, establishing an early diagnosis is critical (2). The diagnosis is established by assessing CSF for the actual trypanosome; however, the changes that the trypanosome causes in the brain can be assessed on the basis of MR findings. MR imaging appears to be an excellent method for assessing and following up those patients suspected of having AF. To date, limited documented brain imaging findings of this disease exist.
Case Report
A 42-year-old man born in the African Congo, but living in Canada since 1998, presented late in 2000 with fatigue, muscular pain, headache, decreased level of consciousness, and increasing somnolence. This man’s medical history was also remarkable for psychiatric problems, such as “hearing voices” and depression. He was known to be seropositive for Epstein-Barr virus, tuberculosis, and hepatitis B.
Physical examination showed a sleepy, febrile man in no acute distress. The examination revealed no organomegaly but did show bilaterally enlarged posterior cervical nodes and enlarged right axillary lymph nodes. His gait was quite bradykinetic, and he was found to have a decreased sensation to light touch on the right side of his body. No other abnormalities were found on physical examination.
Laboratory testing showed normocytic anemia and thrombocytopenia. The white blood cell count was high, with abnormally high lymphocytes and monocytes, but a low neutrophil count. CSF analysis showed an increase in the protein content, but normal levels of glucose. Analysis of CSF also revealed a few trypanosomes. A large right cervical node was excised, and biopsy results indicated nondiagnostic lymphoid hyperplasia.
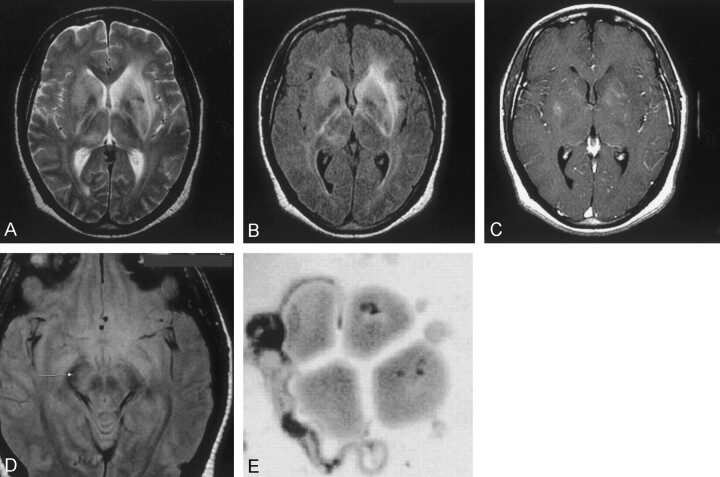
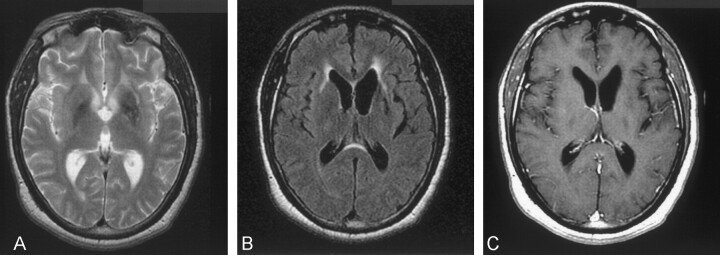
On the day of admission, an MR examination of the brain was performed (Fig 1A-D). Multiplanar T1-weighted, T2-weighted, and fluid-attenuated inversion recovery MR images of the brain with postgadolinium axial and coronal sequences were performed. MR results showed increased signal intensity in the basal ganglia, and hyperintensity in both the periventricular white matter and midbrain. The patient received 100 mg/kg eflornithine every 6 hours during his 2-week hospital stay. There was only slight clinical improvement with this therapy. A 1-year follow-up MR examination demonstrated that areas of basal ganglia white matter hyperintensity had decreased compared with that at initial imaging. It also showed a moderate amount of ventricular enlargement had occurred since the initial MR examination. (Fig 2A–C). This was indicative of white matter loss in the 1-year period.
Fig 1.
Images obtained at time of admission to the hospital.
A, Axial T2-weighted image (TR/TE/NEX, 3000/98/1; section thickness, 5 mm) shows diffuse hyperintensity in both basal ganglia and along the internal, external, and extreme capsules.
B, Axial FLAIR sequence (TR/TE/NEX, 10,002/162.5/1; section thickness, 5 mm) shows bilateral increased signal intensity involving the internal and external capsules bilaterally and optic radiations.
C, Axial T1-weighted gadolinium-enhanced image (TR/TE/NEX, 417/20/2; section thickness, 5 mm) shows minimal enhancement in the basal ganglia bilaterally.
Sahlas DJ, MacLean JD, Janevski J, Detsky AS. Out of Africa. N Engl J Med 2002; 347:749–753. Copyright 2002 Massachusetts Medical Society. All rights reserved.
D, Axial proton density–weighted (TR/TE/NEX, 3000/14/1; section thickness, 5 mm) image shows increased signal intensity in the midbrain. E, Trypanosome adjacent to red blood cells is seen after analysis of the CSF. (Sahlas DJ, MacLean JD, Janevski J, Detsky AS. Out of Africa. N Engl J Med 2002; 347:749–753. Copyright 2002 Massachusetts Medical Society. All rights reserved.) (GIEMSA stain; magnification, ×3000)
Fig 2.
Follow-up images obtained 1 year after initial presentation.
A, Axial T2-weighted image (TR/TE/NEX, 3000/105/1; section thickness, 6 mm) shows notable decrease in signal intensity in the basal ganglia, whereas moderate hyperintensity persists in the left external capsule.
B, Axial FLAIR image (TR/TE/NEX, 10,002/175/1; section thickness, 5 mm) shows minimal hyperintensity extending to both external capsules and demonstrating significant increase in size of the lateral ventricles.
C, Axial T1-weighted gadolinium-enhanced (TR/TE/NEX, 500/20/2; section thickness, 5 mm) image shows no abnormal enhancement; however, notable ventricular enlargement is evident compared with that of initial MR images.
Discussion
More than 50 million people living in 36 African countries are at risk of contracting AT. The trypanosomes that cause this disease are found mostly localized to the west, east, and central regions of Africa. There are 20,000 to 25,000 new cases reported annually in Africa; however, only 31 cases have been reported in the United States in the 20th century (3, 4). It is caused by a flagellated protozoan parasite that is transmitted to humans by the tsetse fly (genus Glossina). The infection in humans is manifested in days, in some cases, and years, in other cases, after a bite from an infected tsetse fly. The disease is caused by the parasite Trypanozoon brucei rhodesiense in eastern Africa and Trypanozoon brucei gambiense in western Africa (Fig 1E) (5).
African trypanosomiasis has many characteristic features, which may aid in diagnosis of the disease. After the initial bite of the tsetse fly, an inflammatory lesion may result in a trypanosomal chancre. As the disease progresses, the parasites multiply and are disseminated throughout the body via the blood stream. Certain specific symptoms occur when the trypanosomes cross the blood-brain barrier. During this second stage, the classic symptoms that give the disease the name “African sleeping sickness” are manifested. Sleeping sickness refers to the somnolence that occurs during the day and the restlessness and insomnia that occur at night. The expression of neurologic symptoms depends on which portion of the brain is affected by the infection. Hyperreflexia, delayed hyperaesthesia, tremors, dyskinesia, slurred speech, unsteady gait, mental confusion, and psychosis all may be experienced by the patient during the course of this disease. Further progression of the disease can result in permanent brain damage and can lead to coma and, eventually, death (3, 6).
CT findings of AT have been documented by several studies. Poisson et al (7) reported diffuse hypoattenuated areas affecting the centra semiovale and periventricular regions. In addition, CT studies conducted by Robinson et al (8) reported the involvement of the basal ganglia. The first case to document the MR findings of meningoencephalic involvement of the central nervous system was reported by Sabbah et al (9). The affected patient in that study demonstrated symmetrical, focal lesions in the deep white matter, the internal capsule, cerebellum, and splenium of the corpus callosum. MR imaging in our case revealed high signal intensity in the area of the basal ganglia. Increased signal intensity was also present in the periventricular white matter of both cerebral hemispheres and within the anterior aspect of the midbrain along the cortical spinal tracts. MR imaging also showed an increase in the ventricular size consistent with an atrophic, mainly subcoritcal, process. In this case, no meningeal involvement occurred, so impairment in CSF resorption did not cause ventriculomegaly. Two routes by which the trypanosomes invade the brain parenchyma have been proposed. One is an early seeding in the choroid plexus and secondary passage into the CSF. The other is direct passage into the cerebral capillaries. Once these vessels are involved, the large extracellular spaces within the white matter allow for movement of the parasite into the brain tissue (10). This latter explanation may explain the lack of meningeal MR imaging findings in our subject, with the presence of white matter findings.
Conclusion
The appearance of CNS trypansomiasis in our case manifested as hyperintense regions within the basal ganglia, internal capsule, external capsule, and extreme capsule, with very little enhancement after contrast material administration. The follow-up examination demonstrated resolution of much of these changes; however, notable atrophy was the main finding.
Acknowledgments
We appreciate the assistance of Dr. Dick MacLean of the Department of Infectious Disease, McGill University.
References
- 1.Gallais P, Badier M. Recherches sur l’encephalite de la trypanosomiase humaine africaine: conclusions cliniques, anatomiques, electroencephalogaphiques, biologiques. Med Trop 1952;12:633–673 [PubMed] [Google Scholar]
- 2.Poltera AA. Pathology of human African trypanosomiasis with reference to experimental African trypanosomiasis and infections of the central nervous system. Br Med Bull 1985;41:169–174 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (1986) Epidemiology and control of African trypanosomiasis. Report of a WHO Expert Committee. Technical WHO Expert Committee. Technical Report Series. No. 739. World Health Organization, Geneva [PubMed]
- 4.Bryan R, Waskin J, Richards F, et al. African trypanosomiasis in American travelers: 20 year review. In: Steffen R, Lobel HO, Haworth J, Bradley DJ, eds. Travel Medicine. Berlin: Springer-Verlag;1989. :384–388
- 5.Leach T. African trypanosomiasis. Adv Vet Sci Comp Med 1973;17:119–162 [PubMed] [Google Scholar]
- 6.Schulzberg M, Ambatsis M, Samuelsson EB, et al. Spread of Trypanosoma brucei to the nervous system: early attack on the circumventricular organs and sensory ganglia. J Neurosci Res 1988;21:56–61 [DOI] [PubMed] [Google Scholar]
- 7.Poisson H, Bleibel J, Regnier R, et al. Formes pseudotumoresles de la trypanosomiasis Africaine a trypanosoma gambiese: etrude etinique et tomodensitometrique. Semaine des Hopitaux.1979. (Paris) 56 [PubMed]
- 8.Robinson B., Clark RM, King JF, et al. Chronic Gambian trypanosomiasis. South Med J 1980;73:516–518 [DOI] [PubMed] [Google Scholar]
- 9.Sabbah P, Brosset C, Imbert P, et al. Human African trypanosomiasis: MRI. Neuroradiology 1997;39:708–710 [DOI] [PubMed] [Google Scholar]
- 10.Mulenga C, Mhlanga JDM, Kristensson K, Robertson B. Trypanosoma brucei brucei crosses the blood-brain barrier while tight junction proteins are preserved in a rat chronic disease model. Neuropathol Appl Neurobiol. 2001;27:77–85 [DOI] [PubMed] [Google Scholar]