Abstract
Summary: Four patients with cerebral arteriovenous malformations (AVMs) underwent superselective Wada testing with intraarterial amobarbital and lidocaine before embolization. In all four patients, the use of lidocaine detected clinically significant neurologic deficits that amobarbital alone did not, likely because of the pharmacodynamic differences of the two agents. The use of lidocaine with amobarbital for superselective Wada testing in patients with cerebral AVMs may improve the sensitivity and predictive value of this test in the future.
The prevention of treatment-related morbidity is crucial to the management of arteriovenous malformations (AVMs), because the natural history of these lesions is still being unraveled, and permanent neurologic deficits following treatment have been reported in approximately 10% of cases (1). Some centers use a modified Wada procedure to detect eloquent brain tissue perfused en passage by feeding arteries and thereby predict any neurologic deficits that might permanently result from embolization or subsequent resection of that particular region. Short-acting barbiturates, such as amobarbital, are generally used for the intraarterial injections, but they selectively inhibit only gray matter structures. At our institution, we routinely perform preembolization Wada testing by using both amobarbital and lidocaine, which inhibits white matter tracts as well.
Technique
All patients were treated at our institution by at least two of the authors between 1998 and 2001 and were known to have cerebral AVMs. Each patient provided informed consent for preembolization superselective Wada testing with amobarbital and lidocaine. Patients were informed that the use of amobarbital and lidocaine in this setting was “off-label,” meaning even though both drugs were approved for multiple clinical indications by the U.S. Food and Drug Administration, neither agent had been specifically approved for use in superselective Wada testing. Nevertheless, their safety and efficacy in superselective Wada testing was supported by the medical literature (2, 3).
All procedures were performed without a formal review by the local institutional review board (IRB), because neither the Wada testing nor the use of amobarbital and lidocaine was investigational, but rather both were components of good medical practice considered to be in the best interest of all patients treated (4). IRB approval was also not requested when making this report, because all observations were made by the reporting physicians during routine clinical practice, no prospective data collection occurred, and no unsolicited, retrospective review of hospital medical records was required.
Under sterile conditions, the right femoral artery was cannulated by using the Seldinger procedure, and a vascular catheter was advanced to the carotid circulation. A Magic (Balt, Montmorency, France) microcatheter was then navigated toward the AVM nidus, and superselectively inserted into a feeding artery chosen for embolization. Wada testing was performed by superselectively injecting amobarbital (50 mg in 1 mL), lidocaine (10 mg in 1 mL), or both, with a contrast agent into the intended feeding artery. Most patients at our institution receive the combination of amobarbital and lidocaine during testing, but either agent may be used separately, at the discretion of the interventional neuroradiologist, to distinguish between cortical and subcortical effects of the anesthetic injections if such a distinction might be useful in planning further treatment.
All patients underwent standardized neurologic assessments immediately before and immediately after each anesthetic injection. The duration of each assessment was intentionally less than 5 minutes, to allow completion of testing within the time of maximum anesthetic effect. All patients received a motor examination that assessed for facial droop, pronator drift within 10 seconds, rapid successive finger-to-thumb tapping, rapid successive foot tapping, and power to confrontation in the deltoids, biceps, wrist extensors, finger extensors, finger flexors, finger abductors, and ankle dorsiflexors and plantar flexors. Power was recorded by using the standard Medical Research Council scale of 0–5. Sensory modalities were also tested in all patients—specifically, light touch, joint position, temperature, and stereognosis—and the development of sensory extinction and subjective paresthesias was recorded.
Assessments of language, memory, and visual-spatial function during the superselective Wada procedures were adapted from well-known neuropsychological tests with prespecified norms (5–7) and were guided by AVM location and arterial supply. Patient 1 had a left anterior temporal AVM with an anterior choroidal artery feeder and, thus, was also evaluated with a test of verbal memory adapted from the Wechsler Memory Examination (5) in which five words were registered and then recalled after 3 minutes. The test was scored on a scale of 0–15, with points assigned for each word recalled. Three points were given for words recalled spontaneously, 2 points for words recalled after a categorical cue, and 1 point for words correctly chosen from a list of four. Patients 2 and 4 had left fontal-parietal AVMs with middle cerebral artery (MCA) feeders and, therefore, were also evaluated with a language battery adapted from the Boston Diagnostic Aphasia Examination (6). Language testing included spontaneous word fluency, two-step commands, comprehension of complex ideational material, picture naming, sentence repetition, and word reading. Patient 3 had a right-sided frontal-parietal AVM and thus was also evaluated for visual-spatial neglect by use of a test of line judgment (7). In this test, 12 lines of various lengths were displayed on a flat computer screen and divided into red and blue segments. The patient was then asked to identify the longer of the two segments or to indicate if they were the same length.
Case Reports
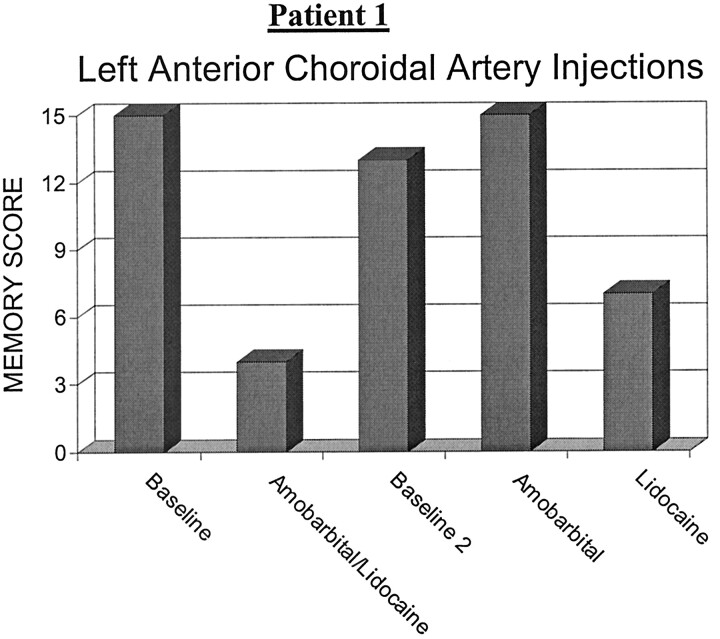
Patient 1 was a 44-year-old right-handed woman with a left anterior temporal AVM that came to clinical attention because of new-onset complex-partial seizures. The AVM was supplied by the left anterior choroidal artery, left lenticulostriate arteries, and left cavernous carotid feeders. Preembolization, superselective Wada testing was performed with a microinterventional catheter placed in a distal branch of the left anterior choroidal artery. Baseline memory testing revealed a memory score of 15. After a superselective injection of amobarbital and lidocaine together, the patient’s memory score declined to 4. After a washout period of 20 minutes following injection, the patient was retested, documenting a return to baseline with a memory score of 13. Subsequently, amobarbital alone was injected into this same distal arterial feeder and produced no deterioration in her memory score from baseline, again being 15. Lidocaine alone was then injected into this same artery and produced a marked decline in her memory score to 7 (Fig 1). The patient again returned to her baseline after a sufficient washout period and suffered no sequelae from the procedure. Motor and sensory testing never deteriorated from baseline. In light of the risk of producing a significant, permanent memory deficit, as suggested by the Wada procedure, no embolization of this distal choroidal feeder was performed.
Fig 1.
Patient 1. Graphic depiction of neurologic performance after anterior choroidal artery injections.
Patient 2 was a 34-year-old right-handed woman who initially presented with a left frontal intracerebral hemorrhage producing a transient hemiparesis. She was found to have a left frontal-parietal AVM supplied by multiple branches of the left MCA, including the rolandic and prerolandic arteries. By using a microcatheter, a left prerolandic branch was cannulated, and preembolization superselective Wada testing was performed. Amobarbital alone was initially injected into this feeding vessel, with no deficits noted on neurologic examination. Lidocaine alone was injected next into this same vessel and produced a temporary paresis of the right hand (MRC = 4 for finger flexion, extension, and abduction). Consequently, this prerolandic feeding artery was not embolized. The rolandic branch was subsequently cannulated, and no motor deficits were produced by superselective injection of amobarbital or lidocaine. Language testing was also performed with each motor examination, and no language deficits were induced by any of the anesthetic injections. As such, embolization of the rolandic branch was accomplished with acrylate glue during induced hypotension, and no new neurologic deficits could be detected after the procedure.
Patient 3 was a 36-year-old right-handed woman with a right frontal-parietal AVM that came to clinical attention after a generalized seizure. Angiography revealed a diffuse nidus fed by two dominant vessels of the MCA, a middle ascending frontal branch and a posterior branch, as well as two branches of the anterior cerebral artery. Superselective intraarterial injection of amobarbital into the middle ascending frontal branch of the MCA resulted in mild weakness of her left thumb only (MRC = 4 for thumb flexion and abduction). Subsequent superselective injection of lidocaine alone into this same branch produced more extensive weakness of the left hand and wrist (MRC = −4 for finger flexion, extension, and abduction; MRC = 4 for wrist extension), as well as a clear left facial droop. Other neurologic assessments, including the test of line judgment, revealed no other deficits. No feeding artery could be found that did not produce significant paresis with anesthetic testing. Therefore, no therapeutic embolizations were performed on this patient.
Patient 4 was a 19-year-old right-handed woman with a large left frontal-parietal AVM that came to clinical attention 4 years earlier, because of progressive neurologic deficits, including memory loss, dyscalculia, and right hypesthesia. Preembolization Wada testing of a large premotor MCA feeder with amobarbital and lidocaine together produced significant weakness of her right arm, with 3/5 power in her hand and 4/5 power at the elbow and shoulder, and significant astereognosis in her right hand. Otherwise, she had no sign of aphasia, and her baseline dyscalculia was unchanged. Twenty minutes post injection, her monoparesis had completely resolved and her examination was again baseline. Subsequently, the catheter was advanced more distally into this same premotor MCA feeder. At this location, we injected amobarbital alone, and detected no face, arm, or leg weakness. We then injected lidocaine alone into this vessel and detected a moderate “pseudoulnar” paresis in her right hand, with 4−/5 weakness of the intrinsic hand muscles of the ring and little finger as well as 4/5 weakness in the flexors and extensors of these two fingers. Her deltoid was also weak at 4+/5 and produced a mild drift, but the remainder of her motor examination was normal. Her sensory and language examination were also normal.
We decided that the benefit of treating this AVM justified embolization of this vessel, despite the anticipated “pseudoulnar” palsy that was likely to develop. Therefore, this vessel was embolized successfully with acrylate glue during induced hypotension, and, as expected, mild weakness developed identical to that seen with the lidocaine injection. This weakness produced only minimal functional impairment in her daily life.
Discussion
In these four cases, focal brain inactivation with lidocaine produced clinical deficits not seen with amobarbital, likely because of the pharmacodynamic differences of the two agents. Amobarbital is a short-acting barbiturate that acts at the gamma-aminoburyric acid A (GABAA) receptor to inhibit postsynaptic neurons throughout the cortical gray matter, hippocampus, basal ganglia, and thalamus. Cerebral white matter, however, is largely free of GABA-ergic synapses and, thus, insensitive to amobarbital inhibition. Lidocaine, by contrast, is a local anesthetic that produces neuronal inactivation by blocking the voltage-gated sodium channels present on all nerve cell membranes, thereby inhibiting both gray and white matter structures.
The duration of observed clinical effect following the intraarterial injection of amobarbital and lidocaine into the nervous system is very brief, generally less than 15 minutes, and appears similar whether these agents are used individually or in combination (2, 3, 8, 9). The pharmacodynamic effect of using these drugs in combination, however, has not been well studied, and the possibility of synergistic mechanisms increasing the magnitude of the observed effect cannot be excluded. To our knowledge, however, the administration of these anesthetic agents together, in both clinical and animal studies, has never been reported to produce a specific, qualitative effect not produced by at least one of the agents individually, thus making the possibility of a unique pharmacodynamic mechanisms resulting from the combination of these agents very unlikely.
We hypothesize that one of two explanations best accounts for the absence of significant amobarbital effect seen in our four patients. First, there may have been an absence of GABAA-receptors in the areas perfused by the superselective injections. Because GABA-receptors are nearly ubiquitous in the synapses of gray matter structures, this explanation requires either that only white matter was perfused or that only the white matter component of the injection contained viable or eloquent neurons. For example, GABA-ergic interneurons are abundant in the motor cortex, and consequently local cortical injections of GABA-agonists effectively abolish movements in experimental animal models (10). Patients 2, 3, and 4, however, did not develop motor deficits after amobarbital injection alone, but developed significant deficits after lidocaine injection. As such, the viable tissue perfused by the anesthetic injections in these cases likely involved predominantly white matter pathways critical for motor control, such as corticospinal, thalamocortical, and prefrontal-rolandic projections.
A second explanation for the lack of amobarbital effect could be that gray matter with viable neurons susceptible to GABAA-inhibition was perfused, but that GABAA-inhibition did not interfere with the behavioral functions tested. Patient 1 had anesthetic injections into the anterior choroidal artery, which supplies numerous structures involved in memory, including the anterior hippocampus, pyriform cortex, uncus, and fimbria. Despite the evidence that nearly all interneurons in the hippocampal formation are GABA-ergic (11), the injection of amobarbital produced no significant memory deficits, which suggests that GABA-mediated inhibition in the hippocampus may not inhibit memory function. In support of this interpretation, experimental injections of GABA-agonists into rat hippocampi actually facilitate memory function, whereas local injections of GABA-antagonists impair it (12). In contrast to amobarbital, the dramatic amnestic effect produced by lidocaine may have resulted from inhibition of the predominant afferent pathways into the hippocampus, such as the perforant pathway from the entorhinal cortex to the dentate gyrus or the cholinergic afferents from the medial septum (11).
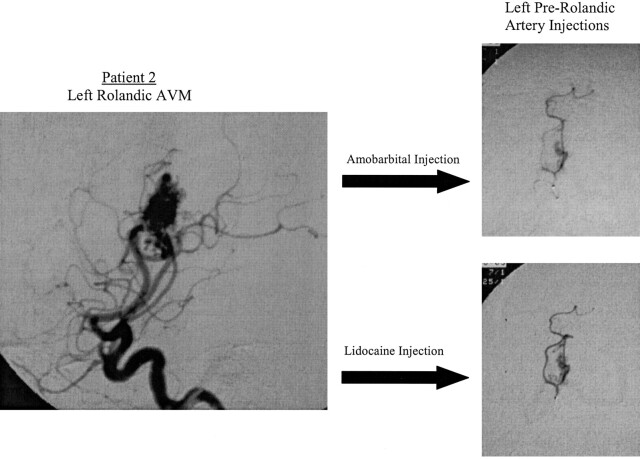
In all four patients, the absence of a response to amobarbital cannot be explained by an overall lack of viable and eloquent neurons in the areas injected, because inhibition of the same areas by lidocaine subsequently produced a clinical deficit. We also do not believe that the amobarbital dose was inadequate, because each vessel received 50 mg of amobarbital, a sizable dose considering the entire carotid territory is usually anesthetized by only 125–150 mg. It is also unlikely that the amobarbital was never delivered to the target tissue, because of AVM shunt dynamics. Shunt surgery of blood flow should affect the amobarbital and lidocaine injections equally, because both drugs were injected in the same volume and at approximately the same rate and both were mixed with contrast agent to confirm identical perfusion patterns radiographically (Fig 2).
Fig 2.
Patient 2. Angiograms of left rolandic AVM (left) and left prerolandic artery after injections (right). Top right image reveals perfusion of amobarbital during superselective injection. Bottom right image reveals the perfusion of lidocaine during superselective injection.
The combination of a local anesthetic and GABAA-agonist for superselective Wada testing is not a novel concept and has been used frequently for extracranial testing (ie, spinal cord [13], eye [14], and face [8]). In 1994, Sadato et al proposed this combination to test patients with cerebral lesions as well and reported a decrease in the incidence of treatment-related morbidity to less than 5% (3); however, this practice never gained wide acceptance, and cerebral AVM embolization procedures are still reported to produce permanent neurologic deficits in approximately 10% of cases overall (1). Many centers do not use preembolization Wada testing at all and instead rely on traditional theories of functional neuroanatomy to predict treatment-related morbidity and guide treatment decisions. The functional anatomy associated with chronic brain lesions, however, is often quite different from traditional paradigms (9, 15), and thus treatment outcomes can be very difficult to predict without the patient-specific information gained from pretreatment superselective Wada testing. In our four patients, even though the geographic areas perfused by individual feeding arteries could be accurately predicted by superselective angiography, the subsequent development or absence of neurologic deficits during Wada testing could not, supporting the importance of preembolization testing in the treatment algorithm for these patients.
Conclusion
Most centers that have incorporated superselective Wada testing into the treatment of AVMs use only GABAA-agonists for intracerebral injection and, therefore, are testing only for gray matter structures. In our series, the coadministration of the nonspecific, local anesthetic lidocaine, to test white matter tracts as well, detected eloquent brain function not revealed by the individual administration of the GABAA-agonist amobarbital. Furthermore, the deficits detected by the addition of lidocaine appear clinically relevant to treatment planning, as demonstrated in patient 4, where therapeutic embolization identically reproduced the deficits predicted by lidocaine testing. Therefore, the use of lidocaine with amobarbital in superselective Wada procedures may increase the sensitivity and predictive value of preembolization testing and thus could potentially reduce the frequency of treatment-related morbidity in patients with AVMs. Further study is required to adequately address these issues in the future.
Footnotes
The authors have no vested interest in the material presented herein.
Presented in part at the 37th Annual Meeting of the American Society of Neuroradiology in Boston, Mass., April 25–27, 2001, and at the 53rd Annual Meeting of the American Academy of Neurology, Philadelphia, Penn., May 5–11, 2001.
References
- 1.The Arteriovenous Malformation Study Group. Arteriovenous malformations of the brain in adults. N Engl J Med 1999;340:1812–1818 [DOI] [PubMed] [Google Scholar]
- 2.Rauch RA, Vinuela F, Dion J, et al. Preembolization functional evaluation in brain arteriovenous malformations: the superselective Amytal test. AJNR Am J Neuroradiol 1992;13:303–308 [PMC free article] [PubMed] [Google Scholar]
- 3.Sadato A, Taki W, Nakahara I, et al. Improved provocative test for the embolization of arteriovenous malformations: technical note. Neurol Med Chir (Tokyo) 1994;34:187–190 [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration Office of Science Coordination and Communication. “Off-label” use of marketed drugs, biologics and medical devices. Information Sheets: Guidance for Institutional Review Boards and Clinical Investigators;1998. update
- 5.Wechsler D. Wechsler Memory Scale: revised manual. San Antonio, TX: Psychological Corporation/Harcourt Brace Jovanovich;1987
- 6.Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination (BDAE). Philadelphia: Lea & Febiger;1983
- 7.Marshall RS, Lazar RM, Krakauer JW, Sharma R. Stimulus context in hemineglect. Brain 1998;121:2003–2010 [DOI] [PubMed] [Google Scholar]
- 8.Deveikis JP. Sequential injections of amobarbital sodium and lidocaine for provocative neurological testing in the external carotid circulation. AJNR Am J Neuroradiol 1996;17:1143–1147 [PMC free article] [PubMed] [Google Scholar]
- 9.Lazar RM, Marshall RS, Mohr JP, et al. Unanticipated memory loss during superselective Wada testing for right cerebral arteriovenous malformation. Stroke 2001;32:337–338 [Google Scholar]
- 10.Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods 1999;86:145–159 [DOI] [PubMed] [Google Scholar]
- 11.Knowles WD. Normal anatomy and neurophysiology of the hippocampal formation. J Clin Neurophysiol 1992;9:252–263 [PubMed] [Google Scholar]
- 12.Castellano C, Cabib S, Puglisi-Allegra S. Psychopharmacology of memory modulation: evidence for multiple interaction among neurotransmitters and hormones. Behav Brain Res 1996;77:1–21 [DOI] [PubMed] [Google Scholar]
- 13.Doppman JL. Girton M, Oldfield EH. Spinal Wada test. Radiology 1986;161:319–321 [DOI] [PubMed] [Google Scholar]
- 14.Horton JA. Dawson RC 3rd. Retinal Wada test. AJNR Am J Neuroradiol 1988;9:1167–1168 [PMC free article] [PubMed] [Google Scholar]
- 15.Lazar RM, Marshall RS, Pile-Spellman J, et al. Anterior translocation of language in patients with left cerebral arteriovenous malformation. Neurology 1997;49:802–808 [DOI] [PubMed] [Google Scholar]