Abstract
It remained inconclusive whether patients with peptic ulcer disease had a higher risk of head and neck cancer (HNC). Therefore, we enrolled 109,360 patients with peptic ulcer disease and matched for age and sex with 218,720 controls from the Taiwan National Health Insurance Research Database between January 1, 1997 and December 31, 2013.The HNC incidence rate was 1.33-fold higher in the peptic ulcer group than in the control group (7.52 vs. 5.68 per 100,00 person-years; crude relative risk: 1.33; 95% confidence interval [CI]: 1.08–1.63) after > 6 years of follow-up. However, in the peptic ulcer subgroup with H. pylori treatment, HNC risk was not significantly different from that of the control group (crude relative risk: 1.12; 95% CI: 0.86–1.46). Moreover, the population with peptic ulcers had the highest risk of laryngeal and hypopharyngeal cancer (adjusted HR: 2.27 [95% CI: 1.16–4.44] and 2.00 [95% CI, 1.13–3.55]), respectively. This observational study suggested that peptic ulcer disease is associated with an increased incidence of laryngeal and hypopharyngeal cancer and H. pylori treatment may have a role in preventing HNC in patients with peptic ulcer disease.
Subject terms: Cancer, Drug discovery, Diseases, Gastroenterology, Health care, Health occupations, Medical research, Risk factors
Introduction
Helicobacter pylori, a spiral-shaped gram-negative bacterium in the aerodigestive tract, is a major cause of peptic and duodenal ulcers1–8. In addition to the lower aerodigestive tract, H. pylori can immigrate to the upper aerodigestive zone9. The human stomach is not the only reservoir of H. pylori, and the bacteria can be observed in the dental plaque, saliva, tonsils, and even adenoid tissue related to gastroesophageal reflux10.
The International Agency for Research on Cancer Working Group (IARC-1994) reported that H. pylori causes gastric cancer and lymphoma3, 11. Because H. pylori can immigrate to the upper aerodigestive zone, several studies have discussed the association between laryngeal or hypopharyngeal cancer and H. pylori12–21. However, results have been inconsistent, and most studies have been single-institute, cross-sectional, or case–control studies rather than long-term cohort studies12–21. Furthermore, H. pylori treatment was associated with a decreased incidence of gastric cancer in a systematic review and meta-analysis22. Currently, no study has discussed whether H. pylori treatment can reduce the incidence of head and neck cancer (HNC).
Taiwan is a newly industrialized country with a rapidly aging population. The prevalence of H. pylori in Taiwan is as high as 50–60%, providing a suitable population for evaluating the relationship between H. pylori and HNC7, 8. To address the aforementioned research gap, this nationwide population base cohort study investigated (1) whether peptic ulcer disease is a risk factor for HNC and (2) whether H. pylori treatment can reduce the risk of HNC in patients with peptic ulcer disease.
Materials and methods
Data source
The National Health Insurance (NHI) program is a single-payer, compulsory, universal health insurance plan established on March 1, 1995 that covers all forms of health care services for more than 99% of Taiwan’s 23.5 million residents23, 24. The National Health Insurance Research Database (NHIRD) included comprehensive medical data, including records of registration, ambulatory and inpatient care, catastrophic illnesses, and drug prescriptions23–27. Patient identification numbers and other sensitive personal data have been encrypted in NHIRD to protect individual privacy27. This study used data from the Longitudinal Health Insurance Database 2000 (LHID 2000), which is a subset of the NHIRD. The LHID 2000 contains detailed information of 1 million randomly selected patients from the 2000 Registry of Beneficiaries of the NHIRD by using a systematic sampling method; moreover, this subset contains all claims data recorded between 1997 and 201328. Data in the LHID 2000 exhibit identical sex and age distribution to that in the NHIRD; therefore, the LHID 2000 is representative of the national patient population29, 30.
This study was approved by the Institutional Review Board of St. Martin De Porres Hospital and waived the requirement for patients’ informed consent as part of the study approval. All methods were carried out in accordance with relevant guidelines and regulations. Diagnostic codes in this study were defined using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
Study design and sample cohort generation
This research was designed to analyze the relationship between H. pylori treatment and subsequent HNC in a cohort of patients with peptic ulcer disease. Patients diagnosed as having various types of peptic ulcers (ICD-9 codes 531-533) and receiving treatment involving proton pump inhibitors (PPIs) between January 1, 1997, and December 31, 2013, were selected from the LHID 2000. PPI treatment was defined as the usage of omeprazole, pantoprazole, lansoprazole, rabeprazole, and esomeprazole. Furthermore, all included patients made at least 2 outpatient service claims or were hospitalized at least once and received a subsequent diagnosis of peptic ulcer disease during the designated period to increase the positive predictive value31. Patients were excluded if they had undergone H. pylori treatment before receiving a diagnosis of peptic ulcers or if they received a diagnosis of HNC or died before the index date. The index date was defined as 90 days after the first diagnosis of peptic ulcer disease. The comparison group (2 patients for every 1 patient with peptic ulcer) was selected using propensity score matching (PSM) for age and sex. All patients in this study were followed until the end of the study period (December 31, 2013) or their voluntary withdrawal from the NHI Program23, 32, 33.
Patients with peptic ulcer were categorized into 2 subgroups on the basis of H. pylori treatment, which was defined as (1) the addition of clarithromycin or metronidazole treatment for 7–14 days within 28 days of PPI treatment, (2) the addition of amoxicillin or tetracycline treatment for 7–14 days within 28 days of PPI treatment, or (3) the combination of the 2 aforementioned with the addition of bismuth for 7–14 days.
Outcome and covariate measurement
The primary outcome in this study was a new diagnosis of HNC. End of the study period (December 31, 2013), voluntary withdrawal from the program, and death were defined as censoring events in this study. The HNC diagnosis was subgrouped on the basis of the following original recorded cancer sites: oral cancer (ICD-9-CM codes 140-145), laryngeal cancer (ICD-9-CM code 161), oropharyngeal cancer (ICD-9-CM code146), hypopharyngeal cancer (ICD-9-CM code148), nasopharyngeal cancer (NPC, ICD-9-CM code147) and nasal and sinus cancer (ICD-9-CM codes160.0-160.9 except 160.1).
Comorbidities were defined using ICD diagnostic codes as at least 1 hospital admission or 2 outpatient visits to treat a given disease within 2 years before the index date34. To increase the representative accuracy of our study, the following comorbid diseases were considered: hypertension (ICD-9 codes 401–405), diabetes mellitus (DM, ICD-9-CM code 250), asthma (ICD-9-CM code 493), chronic obstructive pulmonary disease (COPD, ICD-9-CM codes 490-492 and 493-496), chronic kidney disease (ICD-9-CM code 585), and chronic liver disease (ICD-9-CM codes 571 and 573). Most of these comorbidities were validated in a previous NHIRD study33, 35.
Statistical analysis
PSM was performed for the peptic ulcer and control groups to ensure that baseline characteristics (sex, age, urbanization, length of hospital days, baseline co-morbidity) did not differ significantly between the groups with a standardized difference (SD) of < 0.136, 37. The HNC incidence rate per 10,000 person-years was obtained by dividing the number of patients with HNC by the total person-months at risk. The log-rank test and Kaplan–Meier curves were used to evaluate the cumulative incidence of HNC between the groups. Adjusted hazard ratios (aHRs) for HNC occurrence in each group were estimated using a multiple Cox proportional hazards model. All statistical analyses were conducted using SAS 9.4 (SAS Inc., Cary, NC, USA), and two-sided P < 0.05 was considered statistically significant.
Results
Demographic characteristics
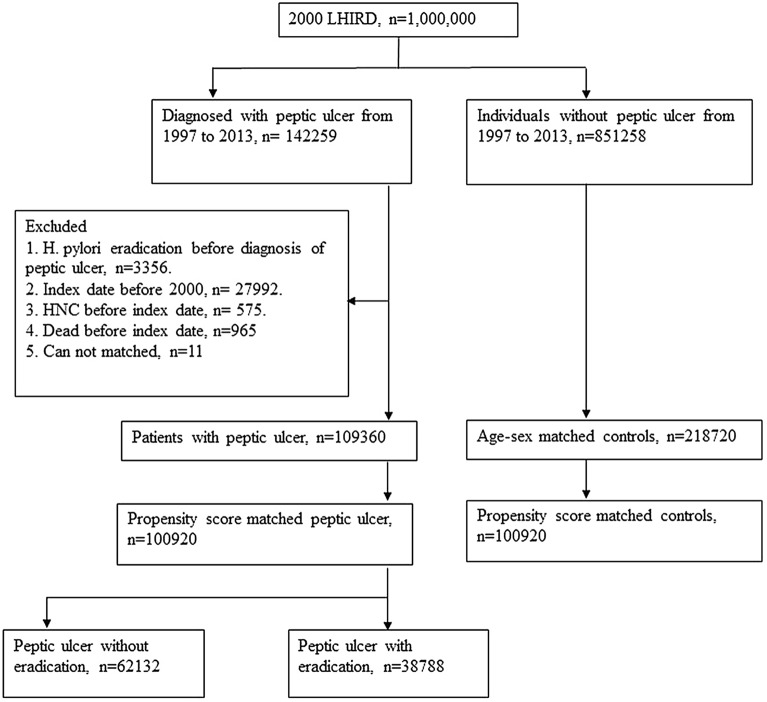
This study included data of 109,360 patients with peptic ulcers and 218,720 controls matched at a 1:2 ratio for age and sex without peptic ulcers (Fig. 1). After PSM to eliminate different baseline characteristics in both the peptic ulcer and comparison groups, each group had 100,920 individuals (Supplementary Table 1). In the peptic ulcer group, 62,132 patients did not undergo H. pylori treatment, and 38,788 patients underwent H. pylori treatment.
Figure 1.
Flow diagram of identification and enrollment of the study patients. LHID = Longitudinal Health Insurance Database; H. pylori., Helicobacter pylori; HNC = Head and Neck Cancer.
The HNC incidence rate in the peptic ulcer and control groups is presented in Table 1. The overall (0–14 years) incidence density rate (per 10,000 person-years) of HNC in the total peptic ulcer and control groups were 7.31 and 6.59, respectively. Although the incidence rate was higher in the total peptic ulcer group, no significant difference was observed between the groups; the crude relative risk was 1.11 (95% confidence interval [CI]: 0.99–1.25). Then, the overall period was divided into 2 groups according to the follow-up periods of 0 to 6 years and > 6 years. In the > 6 years follow up, the incidence of HNC in the control, and total peptic ulcer were 5.68, 7.52, respectively. Compared with the control group, the crude relative risks (95% CIs) of HNC in the total peptic ulcer is significant higher 1.33 (1.08–1.63).
Table 1.
Incidence per 10,000 person-years and crude relative risk for head and neck cancer during 14 years follow-up period.
| Control n = 100,920 |
Peptic ulcer | |||
|---|---|---|---|---|
| Total n = 100,920 |
Untreated n = 62,132 |
Treated n = 38,788 |
||
| Overall period (0–14 years) | ||||
| Observed HNC case | 512 | 584 | 377 | 207 |
| Incidence densitya (95% C.I.) | 6.59(6.04–7.18) | 7.31(6.73–7.92) | 8.04(7.27–8.90) | 6.25(5.46–7.16) |
| Unadjusted hazard ratio (95% C.I.) | Reference | 1.11(0.99–1.25) | 1.22(1.07–1.40) | 0.95(0.81–1.12) |
| Cumulative incidenceb at 3 years (95% C.I.) | 2.01(1.74–2.32) | 2.09(1.82–2.41) | 2.36(1.99–2.80) | 1.67(1.30–2.14) |
| Cumulative incidenceb at 6 years (95% C.I.) | 4.29(3.86–4.77) | 4.30(3.88–4.77) | 4.66(4.10–5.30) | 3.75(3.15–4.46) |
| Cumulative incidenceb at 9 years (95% C.I.) | 5.72(5.19–6.29) | 6.74(6.16–7.37) | 7.54(6.75–8.43) | 5.59(4.80–6.51) |
| Cumulative incidenceb at 12 years (95% C.I.) | 7.69(6.99–8.45) | 8.79(8.05–9.60) | 9.75(8.73–10.88) | 7.44(6.42–8.63) |
| 0–6 years | ||||
| Observed HNC case | 353 | 363 | 237 | 126 |
| Incidence densitya (95% C.I.) | 7.10(6.40–7.88) | 7.18(6.47–7.96) | 7.84(6.90–8.90) | 6.19(5.20–7.37) |
| Unadjusted hazard ratio (95% C.I.) | Reference | 1.03(0.89–1.19) | 1.10(0.94–1.30) | 0.87(0.71–1.07) |
| > 6 years | ||||
| Observed HNC case | 159 | 221 | 140 | 81 |
| Incidence densitya (95% C.I.) | 5.68(4.86–6.62) | 7.52(6.60–8.59) | 8.42(7.14–9.94) | 6.36(5.11–7.91) |
| Unadjusted hazard ratio (95% C.I.) | Reference | 1.33(1.08–1.63) | 1.49(1.18–1.86) | 1.12(0.86–1.46) |
PSM = propensity score matched; HNC = head and neck cancer.
aIncidence density rate, per 10,000 person-years.
bCumulative incidence probability, per 1000 person.
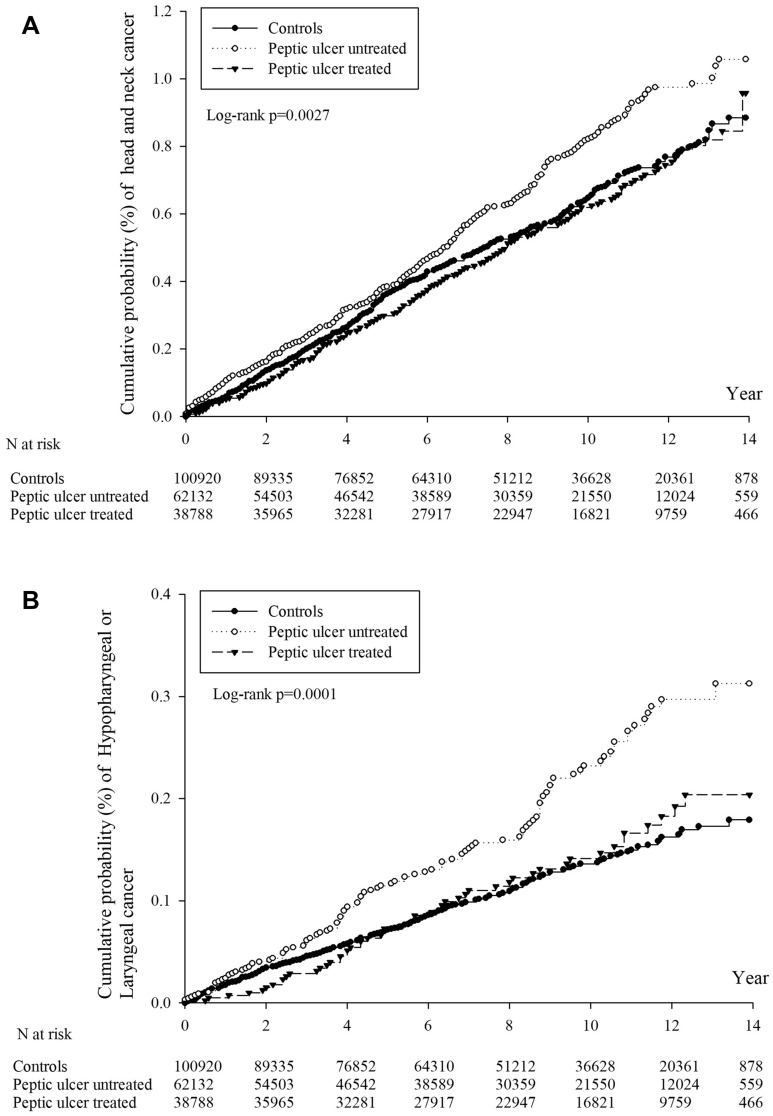
Total peptic ulcer group was divided to 2 groups as “with H. pylori treatment” and “without H. pylori treatment” to eliminate its possible effect of HNC risk. The incidence density of HNC for the overall period in the groups with and without H. pylori treatment was 6.25 and 8.04, respectively. The crude relative incidence of HNC in the groups with and without H. pylori treatment was 0.95 (95% CI: 0.81–1.12) and 1.22 (95% CI: 1.07–1.40) compared with the control group. In > 6 years follow-up period, the incidence of HNC for the overall period in the groups with and without H. pylori treatment can be even upto and 8.42 and 6.36, respectively Then, the crude relative incidence of HNC in the groups with and without H. pylori treatment was 1.12 (95% CI: 0.86–1.46) and 1.49 (95% CI: 1.18–1.86) compared with the control group. The cumulative probabilities of HNC in the PSM population are represented as Kaplan–Meier curves in Fig. 2A (log-rank test, P = .0027).
Figure 2.
(A) The cumulative incidence functions of HNC for peptic ulcer without eradication, peptic ulcer with eradication and controls. (B) The cumulative incidence functions of hypopharyngeal/laryngeal cancer for peptic ulcer without eradication, peptic ulcer with eradication and controls.
The effects of peptic ulcer disease, H. pylori treatment, and covariates on HNC were assessed using the multiple Cox proportional hazards model. Results are presented in Table 2. A significantly different crude relationship emerged between peptic ulcer disease and HNC when follow-up time was > 6 years (aHR: 1.24; 95% CI: 1.01–1.51). Furthermore, when comparing H. pylori treatment policies with the control group, the group without treatment exhibited a significantly higher incidence of HNC than did the control group in the overall period (aHR: 1.23; 95% CI: 1.08–1.40) and after > 6 years of follow-up (aHR: 1.44 95%; CI: 1.16–1.80). However, the incidence of HNC in the group receiving H. pylori treatment was not significantly different from that of the control group in the overall period and the follow-up period of > 72 months. Additionally, sex, age, urbanization, length of hospital stay, and chronic liver disease were possible factors related to HNC. A sensitivity analysis was then performed to adjust possible effects influencing HNC incidence because of differences in follow-up time, sex, and age. The peptic ulcer group without treatment still exhibited a higher incidence of HNC compared with the control group with significant differences in different subgroups (sex and age). The peptic ulcer group with treatment exhibited the lowest incidence of HNC.
Table 2.
Multiple Cox proportional hazard regression for estimation of adjusted hazard ratios on head and neck cancer.
| Variable | Model for peptic ulcer | Model for eradication | ||
|---|---|---|---|---|
| Overall period | ≥ 6 years | Overall period | ≥ 6 years | |
| Study group (ref: control) | ||||
| Peptic ulcer | 1.09(0.96–1.22) | 1.24(1.01–1.51) | ||
| Peptic ulcer without eradication | 1.23(1.08–1.40) | 1.44(1.16–1.80) | ||
| Peptic ulcer with eradication | 0.90(0.76–1.05) | 1.01(0.78–1.31) | ||
| Sex (ref: female) | ||||
| Male | 4.85(4.14–5.68) | 5.08(3.9–6.61) | 4.89(4.17–5.73) | 5.06(3.90–6.57) |
| Age (ref: 30–45) | ||||
| < 30 | 0.22(0.15–0.33) | 0.21(0.12–0.38) | 0.22(0.14–0.32) | 0.20(0.11–0.37) |
| 45–65 | 1.58(1.35–1.84) | 1.12(0.89–1.42) | 1.58(1.36–1.84) | 1.15(0.91–1.45) |
| ≥ 65 | 1.30(1.07–1.58) | 1.11(0.81–1.52) | 1.28(1.06–1.56) | 1.06(0.78–1.46) |
| Urbanization (ref: Urban) | ||||
| Sub-urban | 1.17(1.02–1.33) | 1.19(0.95–1.48) | 1.16(1.02–1.33) | 1.17(0.94–1.46) |
| Rural | 1.39(1.17–1.66) | 1.39(1.03–1.87) | 1.38(1.15–1.65) | 1.36(1.01–1.83) |
| Length of hospital staysa (ref: 0 day) | ||||
| 1–6 days | 1.24(1.05–1.47) | 1.10(0.82–1.48) | 1.24(1.04–1.46) | 1.08(0.81–1.45) |
| ≥ 7 days | 1.57(1.32–1.86) | 1.50(1.11–2.03) | 1.53(1.29–1.81) | 1.52(1.13–2.04) |
| Baseline co-morbidity | ||||
| Hypertension | 1.06(0.92–1.22) | 1.06(0.83–1.36) | 1.05(0.91–1.21) | 1.09(0.86–1.40) |
| Diabetes mellitus | 1.08(0.91–1.28) | 1.04(0.76–1.41) | 1.08(0.91–1.27) | 0.99(0.73–1.34) |
| Asthma | 0.94(0.73–1.20) | 1.00(0.65–1.52) | 0.93(0.73–1.20) | 1.04(0.69–1.56) |
| COPD | 1.01(0.85–1.20) | 0.90(0.67–1.21) | 1.01(0.85–1.19) | 0.92(0.69–1.23) |
| Chronic kidney disease | 1.06(0.69–1.62) | 0.38(0.09–1.52) | 1.04(0.68–1.60) | 0.36(0.09–1.44) |
| Chronic liver diseases | 1.39(1.22–1.58) | 1.27(1.01–1.59) | 1.38(1.21–1.58) | 1.29(1.04–1.62) |
COPD, chronic obstructive pulmonary disease.
aLength of hospital stays, was the sum of the days in the hospitalization within 2 years before index date.
We subgrouped HNC on the basis of its origins as follows: oral cancer, oropharyngeal cancer, NPC, laryngeal cancer, and nasal/sinus cancer. We analyzed aHRs for HNC incidence by origin between the peptic ulcer and control groups by using a multiple Cox proportional hazards model. The peptic ulcer group had a significantly higher incidence of hypopharyngeal (aHR: 2.004; 95% CI: 1.130–3.551) and laryngeal cancer (aHR: 2.272; 95% CI: 1.163–4.440) compared with the control group after > 6 years of follow-up (Table 3). Furthermore, the peptic ulcer group without treatment had the highest incidence of hypopharyngeal and laryngeal cancer with aHR (95% CI) reaching 2.790 (1.525–5.104) and 2.858 (1.403–5.824), respectively. However, the HNC incidence in the group with treatment did not differ from that in the control group for all HNC origins. The cumulative probabilities of hypopharyngeal and laryngeal cancer were significantly different among the peptic ulcer with treatment, peptic ulcer without treatment, and control groups (log-rank test, P < .0001), as revealed by Kaplan–Meier curves (Fig. 2B). Otherwise, in order to eliminate the possible cofactors related to HNC in different groups, we arranged the stratified analyses by age, sex, urbanization, and hospitalization at baseline in Table 4. In the sensitivity analysis among all subgroups, the population with untreated peptic ulcer had a significant higher risk of HNC comparing with control group especially in the subgroups with characters as male, < 30 years old, urban and hospitalization at baseline with aHR (95% CI) reaching 1.23(1.07–1.43), 2.57(1.13–5.89), 1.44(1.20–1.72) and 1.60(1.27–2.01), separately.
Table 3.
Adjusted hazard ratio of different origin of head and neck caner (Follow up > 6 years).
| Sub-events | Control | Peptic ulcer | ||
|---|---|---|---|---|
| Total | Without eradication | With eradication | ||
| Laryngeal cancer | Reference | 2.272(1.163–4.440) | 2.858(1.403–5.824) | 1.539(0.647–3.661) |
| Hypopharyngeal cancer | Reference | 2.004(1.130–3.551) | 2.790(1.525–5.104) | 1.121(0.512–2.450) |
| Oral cancer | Reference | 1.154(0.887–1.500) | 1.352(1.006–1.816) | 0.922(0.650–1.308) |
| Oropharyngeal cancer | Reference | 1.383(0.794–2.407) | 1.502(0.799–2.824) | 1.246(0.623–2.492) |
| Nasopharyngeal cancer | Reference | 0.995(0.641–1.543) | 0.940(0.552–1.600) | 1.060(0.617–1.820) |
| Nasal/sinus cancer | Reference | 0.795(0.288–2.193) | 0.787(0.236–2.620) | 0.805(0.213–3.047) |
Table 4.
The sensitivity analysis for the adjusted hazard ratio (95% C.I.) stratified by follow up time, sex and age groups after PSM.
| aHR (95% C.I.) of HCN | ||||
|---|---|---|---|---|
| Control | Peptic ulcer | |||
| Total | Untreated | Treated | ||
| Overall follow up time | ||||
| Sex subgroups | ||||
| Female | Reference | 1.01(0.76–1.35) | 1.20(0.87–1.64) | 0.71(0.46–1.11) |
| Male | Reference | 1.10(0.97–1.25) | 1.23(1.07–1.43) | 0.93(0.78–1.11) |
| Age at index date | ||||
| < 30 years old | Reference | 1.94(0.86–4.35) | 2.57(1.13–5.89) | 0.68(0.15–3.13) |
| 30–44 years old | Reference | 1.16(0.91–1.49) | 1.35(1.02–1.78) | 0.95(0.68–1.32) |
| 45–64 years old | Reference | 1.13(0.96–1.33) | 1.29(1.07–1.55) | 0.95(0.76–1.18) |
| ≥ 65 years old | Reference | 0.89(0.69–1.13) | 0.96(0.73–1.25) | 0.74(0.51–1.08) |
| Urbanization | ||||
| Urban | Reference | 1.21(1.03–1.43) | 1.44(1.20–1.72) | 0.94(0.76–1.17) |
| Sub-urban | Reference | 0.96(0.78–1.19) | 1.04(0.82–1.32) | 0.86(0.65–1.14) |
| Rural | Reference | 0.93(0.68–1.28) | 1.00(0.70–1.42) | 0.83(0.53–1.29) |
| Hospitalization at baseline | ||||
| No | Reference | 0.97(0.84–1.12) | 1.07(0.90–1.26) | 0.85(0.71–1.03) |
| Yes | Reference | 1.39(1.12–1.72) | 1.60(1.27–2.01) | 1.00(0.73–1.38) |
| Follow up > 6 years | ||||
| Sex subgroups | ||||
| Female | Reference | 1.12(0.69–1.81) | 1.31(0.77–2.22) | 0.85(0.43–1.69) |
| Male | Reference | 1.27(1.02–1.58) | 1.48(1.15–1.89) | 1.03(0.77–1.37) |
| Age at index date | ||||
| < 30 years old | Reference | 1.39(0.44–4.39) | 1.88(0.58–6.18) | 0.54(0.06–4.63) |
| 30–44 years old | Reference | 1.54(1.07–2.23) | 1.86(1.24–2.80) | 1.21(0.76–1.93) |
| 45–64 years old | Reference | 1.14(0.85–1.53) | 1.37(0.98–1.91) | 0.91(0.62–1.34) |
| ≥ 65 years old | Reference | 1.07(0.69–1.68) | 1.11(0.68–1.81) | 1.02(0.55–1.86) |
| Urbanization | ||||
| Urban | Reference | 1.29(0.98–1.69) | 1.59(1.17–2.16) | 0.95(0.66–1.37) |
| Sub-urban | Reference | 1.19(0.84–1.69) | 1.37(0.92–2.02) | 0.97(0.61–1.55) |
| Rural | Reference | 1.20(0.71–2.05) | 1.13(0.61–2.10) | 1.31(0.68–2.54) |
| Hospitalization at baseline | ||||
| No | Reference | 1.19(0.94–1.50) | 1.38(1.06–1.80) | 0.98(0.73–1.33) |
| Yes | Reference | 1.37(0.94–2.02) | 1.60(1.05–2.44) | 1.04(0.61–1.79) |
PSM, propensity score matched; HNC, head and neck cancer.
Discussion
According to our literature review, this is the first study to investigate the association between peptic ulcer disease and HNC risk as well as the possible effects of H. pylori treatment. We observed an association between peptic ulcer disease and an increased incidence of laryngeal and hypopharyngeal cancer after follow-up for more than 6 years. Specifically, among patients with peptic ulcer disease, H. pylori treatment significantly reduced the risk of HNC compared with those without H. pylori treatment. Furthermore, HNC incidence in the peptic ulcer group with H. pylori treatment could be as low as that in healthy patients without peptic ulcers.
The relationship between peptic ulcer disease and HNC is crucial because of their complications and substantial effects on quality of life. Kuipers et al. demonstrated that H. pylori infection was diagnosed in as many as 60–100% patients with peptic ulcer disease38. This relationship between peptic ulcer disease and HNC can be largely attributed to the relationship between H. pylori and HNC. However, few studies have addressed the potential role of H. pylori infection in laryngohypopharyngeal carcinoma and have had with conflicting results13, 15, 16, 19–21, 39, 40. Rezaii et al. evaluated H. pylori serology in 70 cases of laryngeal carcinoma, 28 cases of hypopharyngeal carcinoma, and 105 healthy controls20. After a multivariate regression to eliminate other confounding factors, the odds ratio of H. pylori seropositivity reached 11.49 in the laryngopharyngeal cancer group20. However, Morand et al. performed serological tests, rapid urease tests, and quantitative polymerase chain reactions (qPCRs) for 56 patients with HNC and 90 cancer-free controls. In a logistic regression analysis, the rates of neither positive serology nor rapid urease tests were significantly different between the 2 groups (P = .677 and P = .633, respectively)19. Both of these studies were case–control studies with limited case numbers in a single institute. Moreover, they focused on the difference in H. pylori prevalence between patients with HNC and those without cancer. Cohort studies with longitudinal follow-up and large number big data are lacking.
We enrolled 142,259 patients with peptic ulcer disease and 218,720 age- and sex-matched controls from the LHID 2000. After PSM to eliminate possible confounding factors, the peptic ulcer and control groups each included 100,920 cases (Table 1). Incidences of HNC (follow-up period > 6 years) were 5.68, 7.52, 8.42, and 6.36 per 10,000 person-years in the control, total peptic ulcer, peptic ulcer without treatment, and peptic ulcer with treatment groups, respectively after PSM. The crude relative risk (95% CI) of the peptic ulcer group reached 1.33 (1.08–1.63) and that of the peptic ulcer subgroup without H. pylori treatment reached an even higher 1.49 (1.18–1.86). However, in the group with H. pylori treatment, HNC incidence did not differ significantly from that of the control group (crude relative risk: 1.12; 95% CI: 0.86–1.46). The results of this study also revealed that laryngeal and hypopharyngeal cancer were the 2 HNCs most associated with peptic ulcer disease. The aHRs for hypopharyngeal and laryngeal cancer were 2.004 and 2.272, respectively, in the peptic ulcer group with a follow-up period of > 6 years (Table 3). Otherwise, to eliminate the possible cofactors related to HNC, we arranged sensitivity analysis (Table 4). The results revealed that the population with untreated peptic ulcer has a higher risk of HNC comparing with control group in different age, sex or hospitalization at baseline subgroups (Table 4). Especially in the population with factors as male, < 30 years old, urban and hospitalization at baseline, the aHR (95% CI) can reach 1.23(1.07–1.43), 2.57(1.13–5.89), 1.44(1.20–1.72) and 1.60(1.27–2.01), separately. Hospitalization at baseline revealed that these patients had admitted to hospital for further treatment due to severe peptic ulcer or associated complications such as massive bleeding. Therefore, population with hospitalization at base could be regarded as having more severe peptic ulcer or poor general condition comparing with those without hospitalization at baseline. Therefore, H. pylori treatment could play important role for prevention of HNC especially when patients with severe peptic ulcer or having factors as male, < 30 years old, or urban.
Helicobacter pylori is a microaerophilic gram-negative bacterium discovered by Marshall and Warren in 19841 that affects the etiopathogenesis of chronic illnesses and causes cancer in digestive regions41–43. The mechanism by which H. pylori induces gastric cancer is related to its characteristics of causing persistent inflammation and the development of epithelial metaplasia and genetic instability in gastric regions44. Squamous cell carcinoma, which is a dominant cancer type in the head and neck region, is also related to abnormal epithelial proliferation and differentiation. In addition to the stomach, the oral cavity, tonsil tissue, and saliva are reservoirs of H. pylori, which can colonize in the head and neck region via oral and gastric oral routes (gastroesophageal reflux)40. Therefore, H. pylori induces epithelial cell proliferation in the laryngeal mucosa, as it does in the gastric mucosa, eventually causing laryngeal carcinoma12, 40.
H. pylori treatment can reduce progressive inflammation, histologic damage to the gastric mucosa, peptic ulcer occurrence and recurrence, and the risk of gastric cancer44. In a nationwide population-based cohort study of Taiwan, Wu et al. demonstrated that the incidences of gastric ulcer diseases decreased by 42–48% after H. pylori treatment and PPI use between 1997 and 200645. In addition, a meta-analysis by Lee et al. enrolled 20,484 patients with H. pylori treatment and 27,580 patients without H. pylori treatment46. After a total follow-up of 340,255 person-years, the authors revealed a significant reduction in gastric cancer risk by approximately 50%46. Thus, we hypothesized that H. pylori treatment can also reduce progressive inflammation and damage to the laryngeal and pharyngeal mucosa caused by H. pylori that may be related to laryngeal and hypopharyngeal cancer formation. Our results supported this hypothesis that after patients with peptic ulcer disease undergo H. pylori treatment, the risk of HNC can be similar to that in the population without peptic ulcer (crude relative risk: 1.12; 95% CI: 0.86–1.46). However, patients who did not undergo H. pylori treatment had a 1.49-fold higher risk of HNC compared with the population without peptic ulcer (crude relative risk: 1.49; 95% CI: 1.18–1.86).
This study has several unique strengths. First, this study included large study and control groups (109,360 patients with peptic ulcer and 218,720 age- and sex-matched controls) with long-term follow-up (14 years) by using nationwide insurance data (NHIRD). Because the NHI program provides information on 99% of Taiwan’s 23.5 million residents23, 24, we could trace nearly every patient with HNC within the designated period. Second, this was the first cohort study to evaluate the association among peptic ulcer disease, H. pylori, and HNC. Most studies have been cross-sectional studies evaluating the prevalence of H. pylori infection in HNC by using PCRs or other tests. Third, this is the first study to discuss the possible effect of H. pylori treatment in preventing HNC. Our results revealed that H. pylori treatment may have a role in HNC prevention.
This study has several limitations. First, diagnoses of peptic ulcer disease depended on ICD-9 codes recorded in the NHIRD and might have been inaccurate33, 47. Although we added inclusion criteria of (1) anti-acid medication use and (2) at least 2 outpatient department visits or 1 hospitalization to increase diagnostic precision, we lacked physical examination results and endoscopy findings33, 47. Second, information regarding smoking, alcohol intake, and betel nut use, which are crucial confounding factors of HNC, was unavailable in the NHIRD. Third, the proportion of H. pylori infections in the peptic ulcer group was unavailable.
Conclusions
This is the first nationwide population-based cohort study to investigate the association between peptic ulcer disease and HNC as well as the possible role of H. pylori treatment. The risk of laryngeal and hypopharyngeal cancer was significantly higher in patients with peptic ulcer disease. However, H. pylori treatment successfully reduced the risk of HNC. On the basis of these results, physicians should consider H. pylori treatment when prescribing treatments for patients with peptic ulcers.
Supplementary Information
Acknowledgements
The authors would like to thank St. Martin De Porres Hospital, Chiayi, Taiwan and Chung Shan Medical University hospital, Taichung, Taiwan for the assistance with this article. The present work was partially supported by a grant from the Ministry of Science and Technology, Taiwan, R.O.C. under Grants No. MOST-109-2314-B-040 -010 -MY3. This manuscript was edited by Wallace Academic Editing. Po-Hui Wang, MD were equal contribution to Shun-Fa Yang, PhD as co-corresponding author.
Author contributions
Y.-T.L., C.-H.H., M.-C.W., C.‐C.H., P.-H.W., and S.-F.Y. participated in designing the study. Y.-T.L., Y.C.L., J.Y.H., and C.C.H. participated in generating and gathering the data for the study. Y.T.L., C.H.H., and J.-Y.H. participated in the analysis of the data. All authors participated in writing this article. All authors reviewed and approved all versions of the manuscript competing interests.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Po-Hui Wang, Email: wang082160@yahoo.com.tw.
Shun-Fa Yang, Email: ysf@csmu.edu.tw.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-85598-4.
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Sepulveda AR, Graham DY. Role of Helicobacter pylori in gastric carcinogenesis. Gastroenterol. Clin. N. Am. 2002;31:517–553. doi: 10.1016/S0889-8553(02)00012-2. [DOI] [PubMed] [Google Scholar]
- 3.Stolte SEAM. The significanceof Helicobacter pylori in relation to gastric cancer and lymphoma. Eur. J. Gastroenterol. Hepatol. 1995;7:318–321. [PubMed] [Google Scholar]
- 4.Kuipers EJ. Helicobacter pylori and gastric carcinogenesis. Scand. J. Gastroenterol. 1996;218:103–105. doi: 10.3109/00365529609094739. [DOI] [PubMed] [Google Scholar]
- 5.Graham DY. Helicobacter pylori infection is the primary cause of gastric cancer. J. Gastroenterol. Hepatol. 2000;35:90–97. [PubMed] [Google Scholar]
- 6.El-Omar EM, et al. Increased prevalence of precancerous changes in relatives of gastric cancer patients: critical role of H. pylori. Gastroenterology. 2000;118:22–30. doi: 10.1016/S0016-5085(00)70410-0. [DOI] [PubMed] [Google Scholar]
- 7.Wu CY, et al. Clinicopathological significance of MMP-2 and TIMP-2 genotypes in gastric cancer. Eur. J. Cancer. 2007;43:799–808. doi: 10.1016/j.ejca.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Wu MS, Lee WJ, Wang HH, et al. A case-control study of association of Helicobacter pylori infection with morbid obesity in Taiwan. Arch. Intern. Med. 2005;165:1552–1555. doi: 10.1001/archinte.165.13.1552. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama A, et al. Helicobacter pylori, chronic atrophic gastritis, inactive aldehyde dehydrogenase-2, macrocytosis and multiple upper aerodigestive tract cancers and the risk for gastric cancer in alcoholic Japanese men. J. Gastroenterol. Hepatol. 2007;22:210–217. doi: 10.1111/j.1440-1746.2006.04377.x. [DOI] [PubMed] [Google Scholar]
- 10.Kurtaran H, et al. Role of Helicobacter pylori in pathogenesis of upper respiratory system diseases. J. Natl. Med. Assoc. 2008;100:1224–1230. doi: 10.1016/S0027-9684(15)31471-1. [DOI] [PubMed] [Google Scholar]
- 11.Uemura N, Okamoto S. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer in Japan. Gastroenterol. Clin. N. Am. 2000;29:819–827. doi: 10.1016/S0889-8553(05)70149-7. [DOI] [PubMed] [Google Scholar]
- 12.Akbayir N, Basak T, Seven H, Sungun A, Erdem L. Investigation of Helicobacter pylori colonization in laryngeal neoplasia. Eur. Arch. Otorhinolaryngol. 2005;262:170–172. doi: 10.1007/s00405-004-0794-0. [DOI] [PubMed] [Google Scholar]
- 13.Aygenc E, Selcuk A, Celikkanat S, Ozbek C, Ozdem C. The role of Helicobacter pylori infection in the cause of squamous cell carcinoma of the larynx. Otolaryngol. Head. Neck Surg. 2001;125:520–521. doi: 10.1067/mhn.2001.119438. [DOI] [PubMed] [Google Scholar]
- 14.Borkowski G, et al. A possible role of Helicobacter pylori infection in the etiology of chronic laryngitis. Eur. Arch. Otorhinolaryngol. 1997;254:481–482. doi: 10.1007/BF02439987. [DOI] [PubMed] [Google Scholar]
- 15.Grandis JR, Perez-Perez GI, Yu VL, Johnson JT, Blaser MJ. Lack of serologic evidence for Helicobacter pylori infection in head and neck cancer. Head Neck. 1997;19:216–218. doi: 10.1002/(SICI)1097-0347(199705)19:3<216::AID-HED9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Kizilay A, Aydin A, Kalcioglu MT, Ozturan O, Aydin NE. Histopathologic examination for Helicobacter pylori as a possible etiopathogenic factor in laryngeal carcinoma. Chemotherapy. 2006;52:80–82. doi: 10.1159/000091727. [DOI] [PubMed] [Google Scholar]
- 17.Rubin JS, Benjamin E, Prior A, Lavy J. The prevalence of Helicobacter pylori infection in malignant and premalignant conditions of the head and neck. J. Laryngol. Otol. 2003;117:118–121. doi: 10.1258/002221503762624558. [DOI] [PubMed] [Google Scholar]
- 18.Titiz A, et al. The presence of Helicobacter pylori in the larynx pathologies. Auris Nasus Larynx. 2008;35:534–538. doi: 10.1016/j.anl.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Morand GB, et al. Detection of Helicobacter pylori in patients with head and neck cancer: results from a prospective comparative study combining serology, polymerase chain reaction, and rapid urease test. Head Neck. 2016;38:769–774. doi: 10.1002/hed.23958. [DOI] [PubMed] [Google Scholar]
- 20.Rezaii J, et al. Association between Helicobacter pylori infection and laryngo-hypopharyngeal carcinoma: a case-control study and review of the literature. Head Neck. 2008;30:1624–1627. doi: 10.1002/hed.20918. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Yang Y, Zhou L, Tao L. Association between helicobacter pylori infection and carcinoma of the larynx or pharynx. Head Neck. 2016;38 Suppl 1:E2291–E2296. doi: 10.1002/hed.24214. [DOI] [PubMed] [Google Scholar]
- 22.Lee YC, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016;150(5):1113–1124. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 23.Wu CY, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA. 2012;308:1906–1914. doi: 10.1001/2012.jama.11975. [DOI] [PubMed] [Google Scholar]
- 24.Kuo CL, Chen YT, Shiao AS, Lien CF, Wang SJ. Acid reflux and head and neck cancer risk: a nationwide registry over 13 years. Auris Nasus Larynx. 2015;42:401–405. doi: 10.1016/j.anl.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Lin HC, Chao PZ, Lee HC. Sudden sensorineural hearing loss increases the risk of stroke: a 5-year follow-up study. Stroke. 2008;39:2744–2748. doi: 10.1161/STROKEAHA.108.519090. [DOI] [PubMed] [Google Scholar]
- 26.Chang CH, et al. Infection in advanced chronic kidney disease and subsequent adverse outcomes after dialysis initiation: a nationwide cohort study. Sci. Rep. 2020;10:2938. doi: 10.1038/s41598-020-59794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsiao CH, et al. Cataract surgery-related complications in patients with end-stage renal disease—a nationwide population-based study in Taiwan. Sci. Rep. 2020;10:2159. doi: 10.1038/s41598-020-59160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu CF, et al. Increased risk of deep neck infection among HIV-infected patients in the era of highly active antiretroviral therapy—a population-based follow-up study. BMC Infect. Dis. 2013;13:183. doi: 10.1186/1471-2334-13-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang YH, et al. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J. Hepatol. 2015;63:1111–1117. doi: 10.1016/j.jhep.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J. Clin. Oncol. 2012;30:623–630. doi: 10.1200/JCO.2011.36.0917. [DOI] [PubMed] [Google Scholar]
- 31.Lu YT, et al. Chronic rhinosinusitis after radiotherapy in patients with head and neck cancer: a population-based cohort study in Taiwan. Int. Forum Allergy Rhinol. 2020;10:692–697. doi: 10.1002/alr.22526. [DOI] [PubMed] [Google Scholar]
- 32.Tsai ML, et al. Short- and long-term major cardiovascular adverse events in carotid artery interventions: a nationwide population-based cohort study in Taiwan. PLoS ONE. 2015;10:e0121016. doi: 10.1371/journal.pone.0121016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu YS, et al. Incidence of multilevel surgical procedures and readmissions in Uvulopalatopharyngoplasty in Taiwan. JAMA Otolaryngol. Head Neck Surg. 2019;145:803–810. doi: 10.1001/jamaoto.2019.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu MC, et al. Increased risk of systemic Lupus Erythematosus in patients with Helicobacter pylori infection: a nationwide population-based cohort study. Front. Med. (Lausanne) 2019;6:330. doi: 10.3389/fmed.2019.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu CS, Lai MS, Gau SS, Wang SC, Tsai HJ. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS ONE. 2014;9:e112257. doi: 10.1371/journal.pone.0112257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Normand ST, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J. Clin. Epidemiol. 2001;54:387–398. doi: 10.1016/S0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 37.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuipers EJ, Thijs JC, Festen HP. The prevalence of Helicobacter pylori in peptic ulcer disease. Aliment. Pharmacol. Ther. 1995;9(Suppl 2):59–69. [PubMed] [Google Scholar]
- 39.Malekzadeh R, et al. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the northwest of Iran. J. Clin. Pathol. 2004;57:37–42. doi: 10.1136/jcp.57.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Genc R, et al. The role of H. pylori in the development of laryngeal squamous cell carcinoma. Dis. Mark. 2013;35:447–449. doi: 10.1155/2013/950701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blaser MJ. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-J. [DOI] [PubMed] [Google Scholar]
- 42.Selbach M, Moese S, Backert S, Jungblut PR, Meyer TF. The Helicobacter pylori CagA protein induces tyrosine dephosphorylation of ezrin. Proteomics. 2004;4:2961–2968. doi: 10.1002/pmic.200400915. [DOI] [PubMed] [Google Scholar]
- 43.Hou P, et al. Helicobacter pylori vacA genotypes and cagA status and their relationship to associated diseases. World J. Gastroenterol. 2000;6:605–607. doi: 10.3748/wjg.v6.i4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu JY, Lee YC, Graham DY. The eradication of Helicobacter pylori to prevent gastric cancer: a critical appraisal. Exp. Rev. Gastroenterol. Hepatol. 2019;13:17–24. doi: 10.1080/17474124.2019.1542299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu CY, et al. A nationwide population-based cohort study shows reduced hospitalization for peptic ulcer disease associated with H. pylori eradication and proton pump inhibitor use. Clin. Gastroenterol. Hepatol. 2009;7:427–431. doi: 10.1016/j.cgh.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 46.Lee YC, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016;150:1113–1124. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 47.Kim JY, et al. Association of chronic rhinosinusitis with depression and anxiety in a nationwide insurance population. JAMA Otolaryngol. Head Neck Surg. 2019;145:313–319. doi: 10.1001/jamaoto.2018.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.