Abstract
We aimed to investigate the impact of comorbidity burden on mortality in patients with coronavirus disease (COVID-19). We analyzed the COVID-19 data from the nationwide health insurance claims of South Korea. Data on demographic characteristics, comorbidities, and mortality records of patients with COVID-19 were extracted from the database. The odds ratios of mortality according to comorbidities in these patients with and without adjustment for age and sex were calculated. The predictive value of the original Charlson comorbidity index (CCI) and the age-adjusted CCI (ACCI) for mortality in these patients were investigated using the receiver operating characteristic (ROC) curve analysis. Among 7590 patients, 227 (3.0%) had died. After age and sex adjustment, hypertension, diabetes mellitus, congestive heart failure, dementia, chronic pulmonary disease, liver disease, renal disease, and cancer were significant risk factors for mortality. The ROC curve analysis showed that an ACCI threshold > 3.5 yielded the best cut-off point for predicting mortality (area under the ROC 0.92; 95% confidence interval 0.91–0.94). Our study revealed multiple risk factors for mortality in patients with COVID-19. The high predictive power of the ACCI for mortality in our results can support the importance of old age and comorbidities in the severity of COVID-19.
Subject terms: Infectious diseases, Respiratory tract diseases
Introduction
The coronavirus disease (COVID-19), an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, Hubei, China at the end of December 20191 and has rapidly spread worldwide. On March 11, 2020, the World Health Organization (WHO) declared it a pandemic2. As of May 30, 2020, the global death toll from COVID-19 had exceeded 340,000 according to WHO. Nevertheless, clinical data to guide health care professionals or policy makers in their decision-making is still scarce. Against this backdrop, the government of the Republic of Korea decided to share the COVID-19 nationwide claims data for global research3.
Detection of risk factors for mortality is an important component of the strategies for managing COVID-19. This information is all the more important at a time when the demand for critical care is surging and the resources for healthcare are limited4,5. A recent case series suggested old age and comorbidities as risk factors for severity of COVID-196–8. However, information on how the combination of these risk factors affects the severity of COVID-19 is rare. We also thought that a simple but predictable model is needed for effective health care resource allocation for this public health emergency.
The Charlson comorbidity index (CCI) has been validated for predicting mortality in patients9. CCI quantifies the risk of mortality associated with 19 weighted comorbidities, including congestive heart failure, cerebrovascular disease, chronic pulmonary disease, and diabetes, all of which have been reported as prognostic factors of poor outcome in patients with COVID-199. Recently, the CCI score has been reported to be associated with the mortality and disease severity of COVID-1910. In addition to weighting comorbidities, Charlson and colleagues also proposed the age-adjusted CCI (ACCI) by adding 1 point for every decade after 40 years of age11. Considering the importance of aging and comorbidities in the severity of COVID-19, we expect that ACCI could predict the mortality rate for COVID-19.
In this study, we investigated the age- and sex-adjusted odds ratio (OR) of mortality for each comorbidity and the predictive value of mortality provided by the ACCI for patients with COVID-19 from a nationwide claims database in South Korea. Our results provide valuable information for the identification of patients at high risk of critical illness and might need early intensive care.
Results
In this study, 234,427 (male/female [M/F]: 111,947/122,480) patients had received the diagnostic test for COVID-19, and consequently, 7590 of them (3.2%, M/F: 3095/4495) had been confirmed with COVID-19 in South Korea (Fig. 1). Of this, a total of 7157 (94.3%) patients were admitted to the hospital for COVID-19. Among the hospitalized patients, 216 (3.0%) were admitted to the intensive care unit, 127 (1.8%) received mechanical ventilation, and 21 (0.3%) received extracorporeal membrane oxygenation (ECMO). A total of 227 (3.0%, M/F: 121/106) had died (in-hospital death: 218, out-of-hospital death: 9).
Figure 1.
Summary of the dataset of our data. HIRA Health Insurance Review & Assessment Service, KCDC Korea Centers for Disease Control and Prevention.
The associations between the patients’ demographics, clinical characteristics, and death in the all-patients cohort and Daegu–Gyeongbuk region cohort, are shown in Table 1. In the all-patient cohort, the most common comorbidity was hypertension (n = 1463, 19.3%), followed by chronic pulmonary disease (n = 958, 12.6%) and diabetes mellitus (n = 907, 11.9%). The non-surviving patients were significantly older, predominantly male, covered by medical aid, lived in the Daegu–Gyeongbuk region, and were more likely to have a history of all comorbidities. These characteristics were similar for the Daegu–Gyeongbuk cohort. The patients’ demographics, clinical characteristics, and death by sex are shown in Supplementary Table S1.
Table 1.
Baseline characteristics and comorbidity of patients with coronavirus disease (COVID-19). Data are presented as number (%).
| Patients with COVID-19 (n = 7590) | Patients with COVID-19 in the Daegu–Gyeongbuk region (n = 4234) | |||||
|---|---|---|---|---|---|---|
| Survivor | Non-survivor | P-value | Survivor | Non-survivor | P-value | |
| Total number of patients | 7363 (97.0) | 227 (3.0) | 4033 (95.3) | 201 (4.7) | ||
| Sex | < 0.001 | < 0.001 | ||||
| Male | 2974 (40.4) | 121 (53.3) | 1600 (39.7) | 107 (53.2) | ||
| Female | 4389 (59.6) | 106 (46.7) | 2433 (60.3) | 94 (46.8) | ||
| Age, y | 45 ± 19 | 77 ± 11 | < 0.001 | 49 ± 19 | 77 ± 11 | < 0.001 |
| ≤ 9 | 82 (1.1) | 0 (0.0) | 37 (0.9) | 0 (0.0) | ||
| 10–19 | 346 (4.7) | 0 (0.0) | 132 (3.3) | 0 (0.0) | ||
| 20–29 | 1855 (25.2) | 0 (0.0) | 809 (20.1) | 0 (0.0) | ||
| 30–39 | 774 (10.5) | 2 (0.9) | 366 (9.1) | 1 (0.5) | ||
| 40–49 | 1002 (13.6) | 1 (0.4) | 511 (12.7) | 1 (0.5) | ||
| 50–59 | 1489 (20.2) | 14 (6.2) | 873 (21.6) | 13 (6.5) | ||
| 60–69 | 1025 (13.9) | 36 (15.9) | 706 (17.5) | 31 (15.4) | ||
| 70–79 | 523 (7.1) | 67 (29.5) | 386 (9.6) | 58 (28.9) | ||
| ≥ 80 | 267 (3.6) | 107 (47.1) | 213 (5.3) | 97 (48.3) | ||
| Insurance type | < 0.001 | < 0.001 | ||||
| Health insurance | 6773 (92.0) | 185 (81.5) | 3612 (89.6) | 162 (80.6) | ||
| Medical aid | 590 (8.0) | 42 (18.5) | 421 (10.4) | 39 (19.4) | ||
| Cities and province | < 0.001 | |||||
| Daegu–Gyeongbuk region | 4033 (54.8) | 201 (88.5) | ||||
| Other cities and provinces | 3330 (45.2) | 26 (11.5) | ||||
| Comorbidity | ||||||
| Hypertension | 1307 (17.8) | 156 (68.7) | < 0.001 | 919 (22.8) | 135 (67.2) | < 0.001 |
| Diabetes mellitus | 799 (10.9) | 108 (47.6) | < 0.001 | 578 (14.3) | 100 (49.8) | < 0.001 |
| Congestive heart failure | 168 (2.3) | 44 (19.4) | < 0.001 | 127 (3.1) | 40 (19.9) | < 0.001 |
| Cerebrovascular disease | 339 (4.6) | 53 (23.3) | < 0.001 | 281 (7.0) | 48 (23.9) | < 0.001 |
| Liver disease | 566 (7.7) | 42 (18.5) | < 0.001 | 390 (9.7) | 39 (19.4) | < 0.001 |
| Renal disease | 44 (0.6) | 15 (6.6) | < 0.001 | 36 (0.9) | 13 (6.5) | < 0.001 |
| Chronic pulmonary disease | 875 (11.9) | 83 (36.6) | < 0.001 | 569 (14.1) | 71 (35.3) | < 0.001 |
| Cancer | 236 (3.2) | 27 (11.9) | < 0.001 | 156 (3.9) | 24 (11.9) | < 0.001 |
| Charlson comorbidity index (CCI) | 0 (0–1) | 3 (2–4) | < 0.001 | 0 (0–1) | 3 (2–4) | < 0.001 |
| Age-adjusted CCI | 1 (0–2) | 6 (4–8) | < 0.001 | 1 (0–3) | 6 (5–8) | < 0.001 |
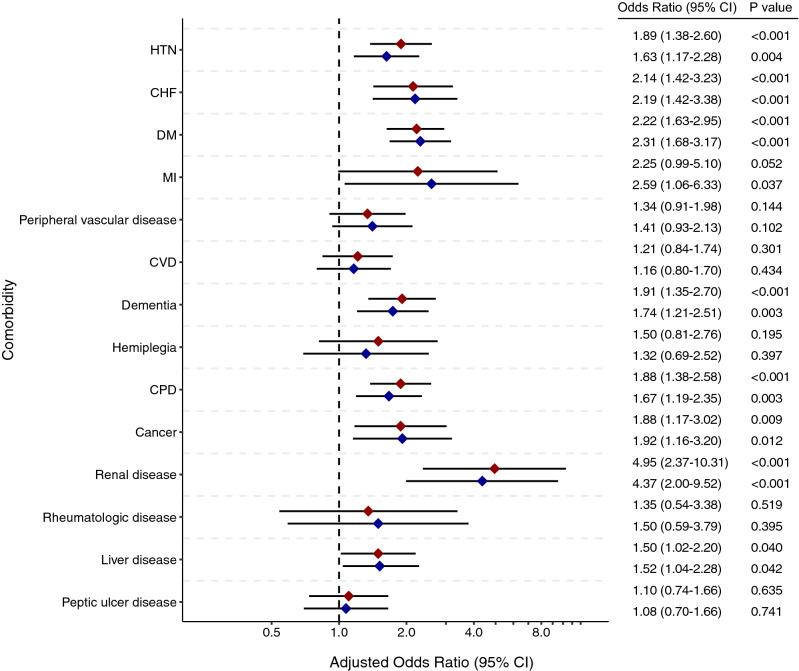
Figure 2 shows the odds ratios (ORs) of mortality by comorbidities with 95% confidence intervals (CIs), with age and sex adjustment in the all-patient and the Daegu–Gyeongbuk cohorts. After adjustment, hypertension (OR 1.89; 95% CI 1.38–2.60), diabetes (OR 2.22; 95% CI 1.63–2.95), congestive heart failure (OR 2.14; 95% CI 1.42–3.23), dementia (OR 1.91; 95% CI 1.35–2.70), chronic pulmonary disease (OR 1.88; 95% CI 1.38–2.58), liver disease (OR 1.50; 95% CI 1.02–2.20), renal disease (OR 4.95; 95% CI 2.37–10.31), and cancer (OR 1.88; 95% CI 1.17–3.02) were significant risk factors for mortality in patients with COVID-19 in the all-patient cohort. A similar result was observed in the Daegu–Gyeongbuk cohort. The significant risk factors in both male and female patients were hypertension, diabetes, and chronic pulmonary disease (Supplementary Fig. S1). In addition, the CCI score was significantly associated with mortality in patients with COVID-19 (Supplementary Table S2).
Figure 2.
Age- and sex-adjusted odds ratios (95% confidence interval [CI]) of mortality according to the comorbidity of the all-patient (red) and Daegu–Gyeongbuk cohorts (blue) with coronavirus disease (COVID-19). CHF chronic heart failure, CPD chronic pulmonary disease, CVD cerebrovascular disease, DM diabetes mellitus, HTN hypertension, MI myocardial infarction. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
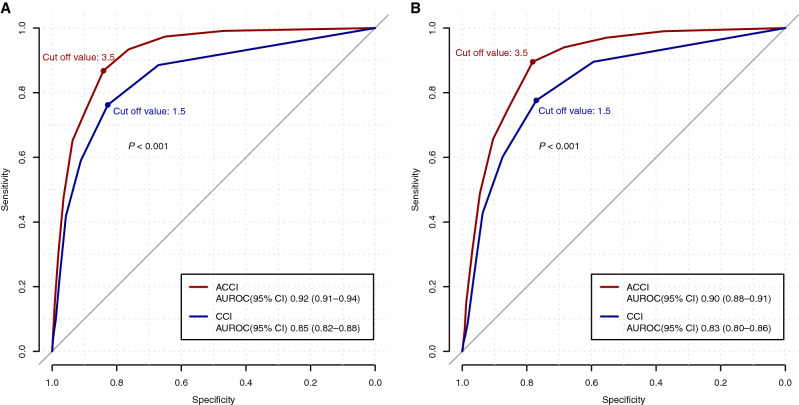
In the all-patient cohort, the receiver operating characteristic (ROC) curve analysis showed that an ACCI threshold > 3.5 yielded the best cut-off point for predicting mortality (area under the ROC (AUROC); 0.92; 95% CI 0.91–0.94) with a corresponding sensitivity of 86.8%, specificity of 84.1%, positive predictive value (PPV) of 14.4%, and negative predictive value (NPV) of 99.5% (Table 2). The predicting performance of the ACCI was superior to that of the original CCI threshold > 1.5 (P < 0.001) (Fig. 3). In the Daegu–Gyeongbuk cohort, an ACCI threshold > 3.5 also yielded the best cut-off point for predicting mortality (AUROC 0.90; 95% CI 0.88–0.91), with a corresponding sensitivity of 89.5%, specificity of 78.2%, PPV of 17.0%, and an NPV of 99.3%. The predicting performance of the ACCI in the Daegu–Gyeongbuk cohort was also superior to that of the original CCI threshold > 1.5 (P < 0.001). The predicting performances of the CCI and ACCI in mortality among patients with COVID-19, according to sex, are shown in Supplementary Table S3 and Supplementary Fig. S2. In male patients, an ACCI threshold > 2.5 yielded the best cut-off point for predicting mortality.
Table 2.
Sensitivity and specificity of the Charlson comorbidity index (CCI) in predicting mortality among patients with coronavirus disease (COVID-19).
| Sensitivity | Specificity | Youden index | PPV | NPV | |
|---|---|---|---|---|---|
| All patients with COVID-19 | |||||
| Age-adjusted | |||||
| CCI ≥ 3 | 0.934 | 0.763 | 1.697 | 0.108 | 0.997 |
| CCI ≥ 4 | 0.868 | 0.841 | 1.709 | 0.144 | 0.995 |
| CCI ≥ 5 | 0.744 | 0.897 | 1.641 | 0.182 | 0.991 |
| Unadjusted | |||||
| CCI ≥ 1 | 0.885 | 0.671 | 1.556 | 0.077 | 0.995 |
| CCI ≥ 2 | 0.762 | 0.828 | 1.590 | 0.120 | 0.991 |
| CCI ≥ 3 | 0.590 | 0.911 | 1.501 | 0.170 | 0.986 |
| Patients with COVID-19 in the Daegu–Gyeongbuk region | |||||
| Age-adjusted | |||||
| CCI ≥ 3 | 0.940 | 0.684 | 1.625 | 0.129 | 0.996 |
| CCI ≥ 4 | 0.895 | 0.782 | 1.677 | 0.170 | 0.993 |
| CCI ≥ 5 | 0.756 | 0.855 | 1.611 | 0.206 | 0.986 |
| Unadjusted | |||||
| CCI ≥ 1 | 0.896 | 0.594 | 1.489 | 0.099 | 0.991 |
| CCI ≥ 2 | 0.776 | 0.771 | 1.547 | 0.144 | 0.986 |
| CCI ≥ 3 | 0.602 | 0.875 | 1.477 | 0.193 | 0.978 |
PPV positive predictive value, NPV negative predictive value.
Figure 3.
The area under the receiver operative characteristic curves of the Charlson comorbidity index (CCI) for predicting the mortality of all the patients (A) and patients living in the Daegu–Gyeongbuk region (B) with COVID-19. The blue and red lines represent the unadjusted and age-adjusted CCI, respectively. ACCI age-adjusted Charlson comorbidity index, AUROC area under the receiver operating characteristic, CI confidence interval. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
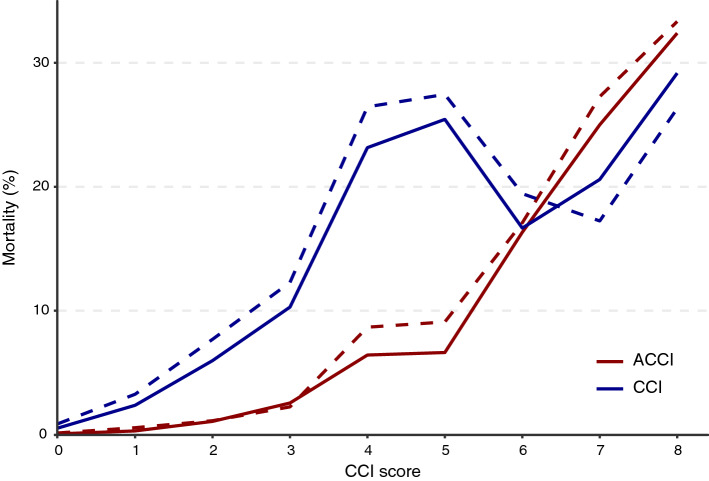
Figure 4 shows the mortality rate according to the CCI and ACCI in the all-patient and Daegu–Gyeongbuk cohorts. The mortality rate showed an increasing trend with the ACCI. The distributions of the CCI and ACCI in the all-patient cohort and Daegu–Gyeongbuk cohorts are shown in Supplementary Table S4. In the all-patient cohort, for those with an ACCI score of 4 or more, the mortality rate was 14.4% (197/1367), and for those with less than 4 points, the mortality rate was 0.5% (30/6223). In the Daegu–Gyeongbuk cohort, for those with an ACCI score of 4 or more, the mortality rate was 17.0% (180/1060), and for those with less than 4 points, the mortality rate was 0.7% (21/3174). Among the 433 non-hospitalized patients, a total of 9 patients had died, and all except 1 patient had an ACCI score of 4 or more (Supplementary Table S5).
Figure 4.
Mortality rate according to the Charlson comorbidity index (CCI) and age-adjusted CCI (ACCI) in the all-patient (solid line) and Daegu–Gyeongbuk cohorts (dotted line).
Discussion
In this study, we investigated the impact of comorbidity burden on mortality in patients with COVID-19 from a nationwide claims database. The main finding of our study was that comorbid hypertension, diabetes, congestive heart failure, chronic pulmonary disease, liver disease, renal disease, dementia, and cancer were identified as significant risk factors for mortality in patients with COVID-19 after age and sex adjustment. The predictive performance of the ACCI was superior to that of the CCI. ACCI > 3.5 was found to be the optimal cut-off value for the prediction of death in patients with COVID-19. Our results can provide useful prognostic information to health care professionals, allowing the selection of patients in most need of medical attention and resources.
The mortality rate reported in our study was lower than that reported in studies conducted in other countries5,12. According to the recent reports from WHO and the Korea Centers for Disease Control and Prevention (KCDC) (last updated June 2, 2020), mortality rates in Europe, Americas, and South-East Asia were 8.4, 5.7, and 2.8%, respectively. These differences may be explained by several factors. One possible explanation for the difference in mortality rates between is the different clinical characteristics of the populations. The risk of COVID-19 mortality has been consistently reported to increase in male patients, patients with an advanced age, and patients with comorbidities, similar to the observations in our study. The patients in our study were relatively younger and had fewer comorbidities than those reported in studies from other countries5,12. These characteristics might be associated with the early COVID-19 outbreak in a relatively large number of young people in South Korea13,14, striking differences between Asian and European mortality might indicate the effect of ethnicity on disease outcome15. However, because ethnicity is a complex entity composed of social constructs, cultural identity, genetic make-up, and behavioral patterns16, it might be difficult to conclude the association between ethnicity and disease outcome. In addition, differences in the organization of health care systems and strategies to contain COVID-19 among different countries may have affected the result. Korea’s rapid and extensive diagnostic testing (more than 10,000 tests daily), and intensive anti-contagion policies may have contributed to the disease outcome17.
In this study, we adjusted both sex and age that could affect the prevalence of comorbidities to investigate its effects on the severity of COVID-19. Further, some studies have suggested that male sex is a risk factor for the severity of COVID-1918,19. It has been suggested that the sex-based difference between the circulating angiotensin-converting enzyme (ACE)-2 levels, the receptor of which was associated with intracellular penetration of SARS-CoV-220, or the smoking rate difference according to sex may have affected the sex difference on the severity of COVID-1919,21. In addition, the potential association between androgen level and COVID-19 severity was suggested22. Our results also revealed the tendency toward sex difference in the mortality of COVID-19. Further research is needed to assess the effect of sex on the severity of COVID-19. Age has consistently been reported to affect the severity of COVID-19 in several studies23,24. In addition to the increased prevalence of comorbidities in older patients25, physiological changes caused by aging itself may affect the severity of COVID-19. Aging leads to the impaired functioning of various systems, including the immune system, resulting in a greater susceptibility to inflammation or death26,27. Frailty, which is commonly associated with the elderly, can also affect the prognosis of COVID-1928. Therefore, we adjusted age and sex in each analysis to investigate the effects of various comorbidities on patient mortality due to COVID-19.
The results from our study revealed multiple risk factors that were associated with mortality in patients with COVID-19 after age and sex adjustment. SARS-CoV-2 binds to the target cells through the ACE-2 receptor expressed in epithelial cells of several organs20. Because the expression of ACE-2 is increased in patients with hypertension, diabetes, and chronic obstructive pulmonary disease (COPD), these comorbidities can increase both the risk and severity of COVID-19 infection29,30. In addition, evidence of myocardial or liver damage has been observed in patients with COVID-19, and pre-existing cardiovascular and liver diseases could be associated with the severity of COVID-19 infection31,32. Recent meta-analyses have identified that cardiovascular diseases and COPD can greatly affect the severity of COVID-1933,34. Renal disease, dementia, and cancer could also be important risk factors for severe COVID-1935–37. An association between these comorbidities and the severity of COVID-19 as defined by other indicators (oxygen therapy, mechanical ventilation, ECMO, and cardiopulmonary resuscitation) has been reported in a recent study from the OpenData4Covid1938. The effects of each comorbidity on the COVID-19 mortality have been observed in our results as well, and if they are combined, the effect will be stronger on the severity of COVID-19.
Our study showed that the ACCI could be more useful in predicting the mortality of patients with COVID-19, compared to the CCI. A study of 52 critically ill patients with COVID-19 revealed that the median duration between the onset of symptoms and intensive care unit admission was 9–10 days, suggesting a gradual progression of the disease39. Therefore, the early detection of risk factors that can predict the severity of disease can improve the patient's prognosis and enable an efficient allocation of medical resources. To this end, we have created a simple but powerful prediction model for the mortality of COVID-19, combining age and comorbidities, known to be important risk factors for the severity of COVID-19. In addition, the high predictive power of the ACCI for mortality in our results could support the importance of old age and comorbidities in the severity of COVID-19. To date, several prognostic models for the severity of COVID-19 have been suggested40,41. The predictive value of ACCI in our study was similar to the recently reported clinical risk score that predicts the occurrence of critical illness in hospitalized patients with COVID-19 (development cohort: area under the receiver operating characteristic [AUROC] 0.88 [95% CI 0.85–0.91], validation cohort: AUROC 0.88 [95% CI 0.84–0.93])40. Therefore, our findings could be useful for healthcare policy-making on the allocation of limited medical resources in the COVID-19 pandemic42,43.
The results of our study should be interpreted cautiously for several reasons. First, the data from insurance claims did not contain detailed clinical information such as vital signs or laboratory values. Although the ACCI showed an excellent predictive value without them, they would have provided other important information regarding the prognosis of COVID-19. Second, the CCI does not consider the use of drugs and relies on diagnosis codes only; thus, over- or underestimation of the risk is likely to have happened. Third, due to the different medical situations and resources for the COVID-19 crisis in each country, the generalizability of the results may be limited. However, the contribution of this study is that it uses nationwide data to provide predictions on the risk of mortality, which is the most serious outcome of COVID-19 infection. Finally, the original ACCI did not include hypertension, which is the most common comorbidity in patients with COVID-19. The development of a COVID-19-specific comorbidity scoring system will be necessary.
In conclusion, our study identified that the ACCI, combined with age and various comorbidities, was associated with mortality in patients with COVID-19 in South Korea. If an increasing number of patients with COVID-19 develop severe illness, plans should be made at the national level to better manage the surge and ensure the need for critical care resources. Furthermore, because the availability of medical resources for critical care is likely restrictive, resource allocation policies based on risk factors should be implemented by medical professionals and policy makers. We hope that our study findings will provide important information to guide health care professionals, who are facing the global health threat of COVID-19, in timely decision-making.
Methods
This study was reviewed and approved by the institutional review board (IRB) of Seoul National University Hospital (IRB No. E-2004-165-1119), and the requirement of informed consent was waived because the data did not contain any identifiable information. All methods were performed in accordance with the approved guidelines and regulations44.
This retrospective cohort study analyzed data from the health insurance claims relevant to COVID-19, submitted to the Health Insurance Review and Assessment (HIRA) of South Korea by May 15, 2020. The HIRA currently provides the data on its website under the project OpenData4Covid19 (https://hira-covid19.net/)3. The current dataset includes a total of 234,427 patients who visited the hospital for the diagnosis of COVID-19 and their health insurance claims between January 1, 2017 and May 15, 2020. The dataset was merged with the COVID-19 confirmation and mortality data from KCDC. Given the obligatory nature of the National Health Insurance system (NHIS), our data covers virtually all Koreans (about 50 million people) and captures the clinical data from all healthcare institutions, i.e., clinics, pharmacies, and hospitals45. Each healthcare institution must submit all patients’ information regarding diagnosis, treatment, medical services rendered, and drug prescriptions to the NHIS, to receive reimbursement46.
In South Korea, COVID-19 diagnostic tests, SARS-CoV-2 real-time polymerase chain reaction is mostly used for persons who have been in contact with COVID-19-positive patients or persons with symptoms that are suspicious of COVID-1947,48. We defined the confirmation of COVID-19 using the confirmation code provided by the HIRA, based on the KCDC data3. Information on the demographics of participants, type of insurance (health insurance and medical aid), and comorbidities was based on claims codes. Patients with comorbidities were defined as those who had more than three claim records between January 2019 and before COVID-19 testing. This was done to avoid over-estimating the comorbidities and to include only those comorbidities that could probably affect the patient’s recent medical condition. The ACCI values were drawn from the claims data using the International Statistical Classification of Diseases and Related Health Problems, 10th edition (ICD-10) coding algorithm proposed by Quan et al.49. The algorithm was applied and validated for the national health insurance claims data in South Korea50,51. The ACCI is a weighted measure that incorporates age into the original CCI11. In the ACCI, an additional point is added for each decade after 40 years of age (from 1 point for the age group 50–59 years to 4 points for the age group greater than 80 years old). Primary hypertension (ICD-10 code I10.x), which is not included in the CCI, was also identified by the aforementioned method.
The main outcome of our study was patient death due to COVID-19. We did not investigate the effects of the comorbidities on the infection rate or the hospitalization rate of COVID-19. We thought that both medical and non-medical conditions, such as the capacity of the medical resources or social factors in the area, could have affected the infection rate or hospitalization rate of COVID-19. For example, the mass outbreaks in some specific groups in South Korea occurred mainly in young age groups with active social activities13,52. These occurrences may result in bias on the medical conditions of the patients with COVID-19.
We performed subgroup analysis for patients with COVID-19 in the Daegu and Gyeongbuk regions with a population of about five million, where most confirmed cases of COVID-19 were reported in South Korea. In South Korea, there has been a surge in the number of confirmed COVID-19 cases in a religious sect gathering called the Shincheonji53. This rapid spread has led to a shortage of hospital beds and healthcare professionals in this area54. We thought that the situation in this region better reflected the current crisis in other countries with a high COVID-19 prevalence. In addition, we performed a comparative analysis between male and female patients to investigate the effect of sex on COVID-19 mortality.
Statistical analysis
The baseline characteristics are presented as mean ± standard deviation for continuous variables and as frequencies with percentages for categorical variables. Continuous variables were compared using unpaired Student’s t-test, and categorical variables were compared using either the chi-square test or Fisher’s exact test, as appropriate. We calculated the age- and sex-adjusted ORs of mortality by comorbidities included in the CCI, using the logistic regression model. For age adjustment, age was classified into ten-yearly age groups (0–9, 10–19, 20–29, 30–39, …years) as a categorical variable. We also performed a multivariable logistic regression analysis with the CCI score as the covariate. The ability of the CCI and ACCI to predict in-hospital mortality among all patients with COVID-19 or hospitalized patients with COVID-19 was determined using the ROC curves and their AUROC. To evaluate the predicting performance, we calculated the AUROC, sensitivity, specificity, PPV, and NPV. DeLong's test was used to compare the ROC curves. All statistical tests were performed with SAS, version 9.4 (SAS Institute; Cary, NC), or R software version 3.6.3 (R Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/).
Supplementary Information
Acknowledgements
The authors appreciate the healthcare professionals who are dedicated to treating patients with COVID-19 in Korea, and the Ministry of Health and Welfare and the Health Insurance Review & Assessment Service of Korea for sharing invaluable national health insurance claims data in a prompt manner. The authors also appreciate Editage (www.editage.co.kr) for the English language editing.
Author contributions
S.I.C. and S.Y. equally contributed to this work as co-first authors. S.I.C. contributed to data curation, statistical analysis, and manuscript revision. S.Y. contributed to study design and manuscript preparation. H.J.L. contributed to study design, manuscript preparation, and manuscript revision.
Funding
The authors have no sources of funding to declare for this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Soo Ick Cho and Susie Yoon.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-85813-2.
References
- 1.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Director-General's opening remarks at the media briefing on COVID-19—11 March 2020.
- 3.Ministry of health and welfare, health insurance review and assessment service, Republic of Korea. Guidelines for COVID-19 international research co-hosted by MoHW and HIRA of Korea version 1.0. https://hira-covid19.net/. Accessed 2 June 2020.
- 4.Rosenbaum L. Facing Covid-19 in Italy—Ethics, logistics, and therapeutics on the epidemic’s front line. N. Engl. J. Med. 2020;382:1873–1875. doi: 10.1056/NEJMp2005492. [DOI] [PubMed] [Google Scholar]
- 5.Grasselli G, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan WJ, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JJ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 8.Yanga J, Zhenga Y, Goua X. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Int. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.Tuty Kuswardhani RA, Henrina J, Pranata R, Anthonius Lim M, Lawrensia S, Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2020;14:2103–2109. doi: 10.1016/j.dsx.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 12.Richardson S, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HJ, et al. The delay in COVID-19 confirmation among cases from a religious group in South Korea. J. Prev. Med. Public Health. 2020;53:164–167. doi: 10.3961/jpmph.20.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudley JP, Lee NT. Disparities in age-specific morbidity and mortality from SARS-CoV-2 in China and the Republic of Korea. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pareek M, et al. Ethnicity and COVID-19: An urgent public health research priority. Lancet. 2020;395:1421–1422. doi: 10.1016/S0140-6736(20)30922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee C. “Race” and “ethnicity” in biomedical research: How do scientists construct and explain differences in health? Soc. Sci. Med. 2009;68:1183–1190. doi: 10.1016/j.socscimed.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Hsiang S, et al. The effect of large-scale anti-contagion policies on the COVID-19 pandemic. Nature. 2020 doi: 10.1038/s41586-020-2404-8. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: Findings of 487 cases outside Wuhan. Crit. Care. 2020;24:1–4. doi: 10.1186/s13054-019-2683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin J-M, et al. Gender differences in patients with COVID-19: Focus on severity and mortality. Front. Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94:e00127-20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir. Med. 2020;8:E20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goren A, et al. A preliminary observation: Male pattern hair loss among hospitalized COVID-19 patients in Spain—A potential clue to the role of androgens in COVID-19 severity. J. Cosmet. Dermatol. 2020;19:1545–1547. doi: 10.1111/jocd.13443. [DOI] [PubMed] [Google Scholar]
- 23.Verity R, et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect. Dis. 2020;6:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2002;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 25.Fabbri E, Zoli M, Gonzalez-Freire M, Salive ME, Studenski SA, Ferrucci L. Aging and multimorbidity: New tasks, priorities, and frontiers for integrated gerontological and clinical research. J. Am. Med. Dir. Assoc. 2015;16:640–657. doi: 10.1016/j.jamda.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Márquez EJ, et al. Sexual-dimorphism in human immune system aging. Nat. Commun. 2020;11:1–17. doi: 10.1038/s41467-020-14396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pranata R, et al. Clinical frailty scale and mortality in COVID-19: A systematic review and dose-response meta-analysis. Arch. Gerontol. Geriatr. 2020;93:104324. doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung JM, et al. ACE-2 Expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2002;55:2000688. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 32.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020 doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 33.Li B, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Lancet Respir. Med. 2020;167:105941. doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020;52:1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bianchetti A, et al. Clinical presentation of COVID19 in dementia patients. J. Nutr. Health Aging. 2020;24:560–562. doi: 10.1007/s12603-020-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID-19 for cancer patients. Lancet Oncol. 2020;21:e180. doi: 10.1016/S1470-2045(20)30150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji W, et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: A nationwide case-control study. J. Korean Med. Sci. 2020;35:e237. doi: 10.3346/jkms.2020.35.e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wynants L, et al. Prediction models for diagnosis and prognosis of COVID-19 infection: Systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang W, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emanuel EJ, et al. Fair allocation of scarce medical resources in the time of COVID-19. N. Engl. J. Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 43.White DB, Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. JAMA. 2020;323:1773–1774. doi: 10.1001/jama.2020.5046. [DOI] [PubMed] [Google Scholar]
- 44.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD). The TRIPOD statement. Circulation. 2015;131:211–219. doi: 10.1161/CIRCULATIONAHA.114.014508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J, Yoon S, Kim LY, Kim DS. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: Strengths, limitations, applications, and strategies for optimal use of HIRA data. J. Korean Med. Sci. 2017;32:718–728. doi: 10.3346/jkms.2017.32.5.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheol Seong S, et al. Data resource profile: The national health information database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park S, Choi GJ, Ko H. Information technology—based tracing strategy in response to COVID-19 in South Korea—Privacy controversies. JAMA. 2020 doi: 10.1001/jama.2020.6602. [DOI] [PubMed] [Google Scholar]
- 48.Hong KH, et al. Guidelines for laboratory diagnosis of coronavirus disease 2019 (COVID-19) in Korea. Ann. Lab. Med. 2020;40:351–360. doi: 10.3343/alm.2020.40.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quan H, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 50.Kim KH. Comparative study on three algorithms of the ICD-10 Charlson comorbidity index with myocardial infarction patients. J. Prev. Med. Public Health. 2010;43:42–49. doi: 10.3961/jpmph.2010.43.1.42. [DOI] [PubMed] [Google Scholar]
- 51.Seo HJ, et al. A comparison of the Charlson comorbidity index derived from medical records and claims data from patients undergoing lung cancer surgery in Korea: A population-based investigation. BMC Health Serv. Res. 2010;10:236. doi: 10.1186/1472-6963-10-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park SY, et al. Coronavirus disease outbreak in call center, South Korea. Emerg. Infect. Dis. 2020 doi: 10.3201/eid2608.201274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi H, Cho W, Kim MH, Hur JY. Public health emergency and crisis management: Case study of SARS-CoV-2 outbreak. Int. J. Environ. Res. Public Health. 2020;17:3984. doi: 10.3390/ijerph17113984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park PG, Kim CH, Heo Y, Kim TS, Park CW, Kim CH. Out-of-hospital cohort treatment of coronavirus disease 2019 patients with mild symptoms in Korea: An experience from a single community treatment center. J. Korean Med. Sci. 2020;35:e140. doi: 10.3346/jkms.2020.35.e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.