Abstract
To overcome technical challenges associated with the use of DNA strand-displacement circuits in vivo, including degradation by cellular nucleases, researchers are increasingly turning to bio-orthogonal l-DNA. Although enhanced stability and improved performance of l-DNA-based circuits within living cells is often implied, direct experimental evidence has not been provided. Herein, we directly compare the functional stability and kinetics of d-DNA and l-DNA strand-displacement in live cells for the first time. Compared to conventional d-DNA, we show that l-DNA strand-displacement reaction systems have minimal “leak”, fast reaction kinetics, and prolonged stability inside living cells. Furthermore, using “heterochiral” strand-displacement, we demonstrate that bio-stable l-DNA reaction components can be easily interfaced with native DNA inside cells. Overall, our results strongly support the broader adoption of l-DNA in the field of DNA molecular circuitry, especially for in vivo applications.
Keywords: strand-displacement reaction, l-DNA, peptide nucleic acid, heterochiral DNA circuit
Graphical Abstract
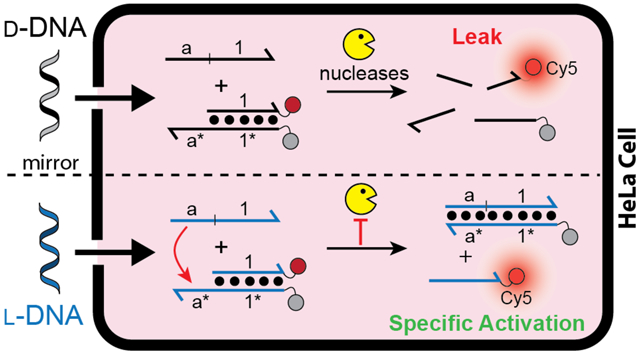
Chemical circuits based on DNA strand displacement have emerged as a promising approach for carrying out molecular computation.1, 2 In particular, the ability to interface DNA-based circuits with endogenous nucleic acids through simple Watson-Crick (WC) base pairing allows for programmable interrogation and/or manipulation of living systems, thereby providing numerous opportunities in research and medicine.3 However, the practical applications of DNA strand-displacement circuits within living cells have been impeded by the intrinsic drawbacks of DNA, including susceptibility to nuclease degradation, protein binding, and off-target hybridization to endogenous nucleic acids, all of which erode circuit performance. In order to overcome these limitations, we (and others) have proposed constructing strand displacement circuits using mirror image l-oligonucleotides.4–6 Due to the inverted stereochemistry of the (deoxy)ribose moiety, l-oligonucleotides are mostly orthogonal to the stereospecific environment of natural biology. Consequent, l-DNA and l-RNA are completely resistant to nuclease degradation, less susceptible to non-specific interactions with cellular macromolecules, and nonimmunogenic.7 Furthermore, because oligonucleotides of opposite chirality (d versus l) are incapable of forming contiguous WC base pairs with each other8, 9, l-oligonucleotides avoid off-target hybridization with abundant cellular nucleic acids. Thus, use of l-oligonucleotides in the design and construction of strand-displacement circuits circumvents many of the key obstacles for utilizing this promising technology in cells. Towards this goal, several groups have successfully employed l-DNA and l-RNA-based biosensors in living cells, supporting the viability of the mirror-image approach.10–13 Nevertheless, while the enhanced stability and improved performance of l-DNA/RNA-based circuits and other nanodevices within cells is often implied, direct experimental evidence is lacking. Herein, we directly compare the functional stability and kinetics of d-DNA and l-DNA strand-displacement systems in live cells for the first time, and demonstrate unequivocally that l-DNA-based stand-displacement circuits have dramatically improved intracellular performance and reliability compared to their native counterparts.
RESULTS AND DISCUSSION
We first compared the stability of d-DNA and l-DNA in cells using a “reporter” complex (d/l-R) as the basis for the measurement (Figure 1a and Figure S1). The reporter consists of DNA duplex modified with a fluorophore (Cy5) and quencher on complement strands and a 5′ single-stranded toehold domain (a*) that enables strand displacement to occur in the presence of an invading strand (d/l-Out). For complex circuits, d/l-Out would be released as the species whose concentration would be measured by the reporter. Thus, the reporter provides the critical optical signal for many biosensing applications of DNA strand-displacement. We transfected HeLa cells with either the d-DNA or the l-DNA version of the reporter complex (d-R and l-R, respectively) and monitored the fluorescence over time using flow cytometry (Figure 1b and Figure S2). Following transfection of d-R into cells, a gradual increase in fluorescence was observed, which nearly reached the maximum obtainable signal after 10 hours based on an unquenched control (Figure S3). As no invading strand was provided, activation of the d-R must be attributed to nuclease degradation and/or non-specific dehybridization of the complex in cells. In contrast, l-R showed a negligible increase in fluorescence over the same period, indicating that l-DNA duplex will remain intact within cells for extended periods of time. Interestingly, both d-R and l-R were mostly resistant to degradation in cell medium (DMEM) supplemented with 10% fetal bovine (Figure S4), suggesting that the intracellular environment contains unique features capable of destabilizing d-DNA duplexes but not their l-DNA counterparts.
Figure 1.
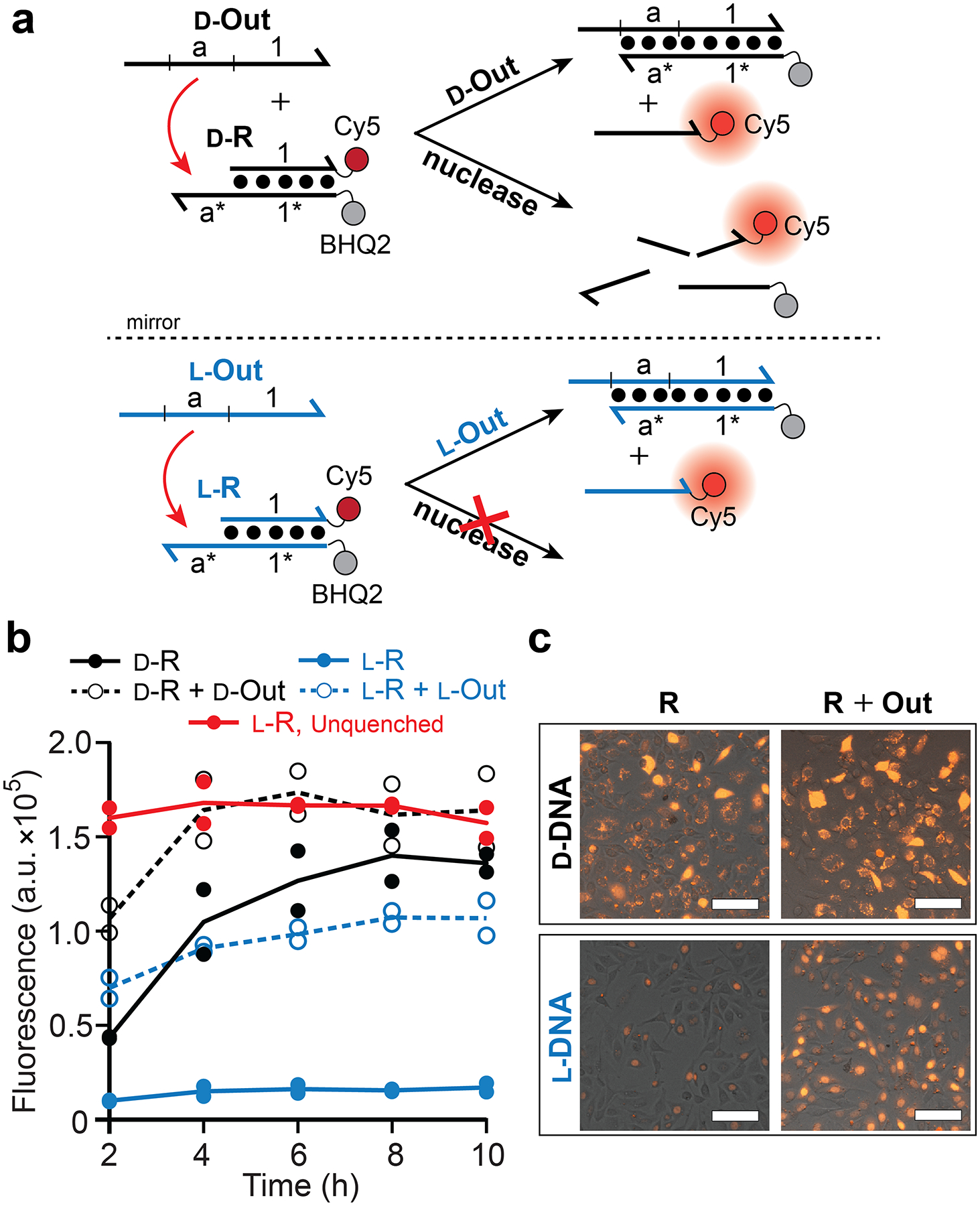
Stability of d-DNA and l-DNA reporter complexes in live cells. (a) Schematic illustration of reporter complexes and their activation by specific and nonspecific pathways. d-DNA (black) and l-DNA (blue) are distinguished by color and are depicted as lines with the half arrow indicting the 3′ end. Sequences of all strands are listed in Table S1. (b) Fluorescent monitoring of reporter complexes in live cells by flow cytometry. Cells were transfected with the indicated components and the first measurement was acquired after 2 h. Red line indicates the unquenched l-DNA reporter control (Figure S3). Individual data points from at least two independent experiments are plotted and connected by the mean. (c) Representative fluorescent microscopy images of HeLa cells following 10 h incubation with the indicated components. Scale bars are 100 μm.
We next compared the functional operation of d-DNA and l-DNA reporter complexes in the presence of an invading strand (d-Out and l-Out, respectively; Figure 1a). To ensure that the strand-displacement reaction occurred within cells, the reporter complex and invader were packaged into separate liposome complexes then delivered simultaneously.14 We confirmed that minimal strand displacement occurred prior to transfection (Figure S5). In the presence of l-Out, intracellular activation of l-R occurred rapidly, resulting in a > 6-fold enhancement in fluorescence after only 4 hours (Figure 1b,c). In contrast, due to high background fluorescence resulting from degradation, the d-R was unable to achieve greater than ~2-fold fluorescence enhancement in the presence of d-Out.
To test a more complex operation in cells, we designed a multi-component reaction cascade that required two successive strand-displacement reactions to activate a fluorescence response (Figure 2a). We employed a photocaged d-DNA input strand (d-IN) that allows for toehold-mediated strand displacement to be triggered by UV light at a defined time point.15 This approach further ensured that the reactions occur within cells and allowed for precise monitoring of intracellular reaction kinetics. In order for the l-DNA reaction configuration to utilize d-IN, the strand containing the toehold domain (t) within complex d-A was substituted completely by a strand of achiral PNA. The resulting PNA-DNA heteroduplex (l-A), which we refer to as an “inversion gate”, effectively inverts the stereochemistry of domain a from d to l, thereby allowing d-IN to be sequence-specifically interfaced with l-R despite their opposing stereochemistry.4 This “heterochiral” approach not only eliminates input stability as a potential variable between the two reaction configurations, but also provides an important proof-of-principle for interfacing endogenous d-nucleic acids with bio-orthogonal l-DNA circuits in live cells. In that regard, d-IN serves as a proxy for nucleic acid biomarkers, such as mRNAs or microRNAs, that could eventually be interfaced with more complex l-DNA-based circuits for applications in bioimaging and disease diagnosis.
Figure 2.
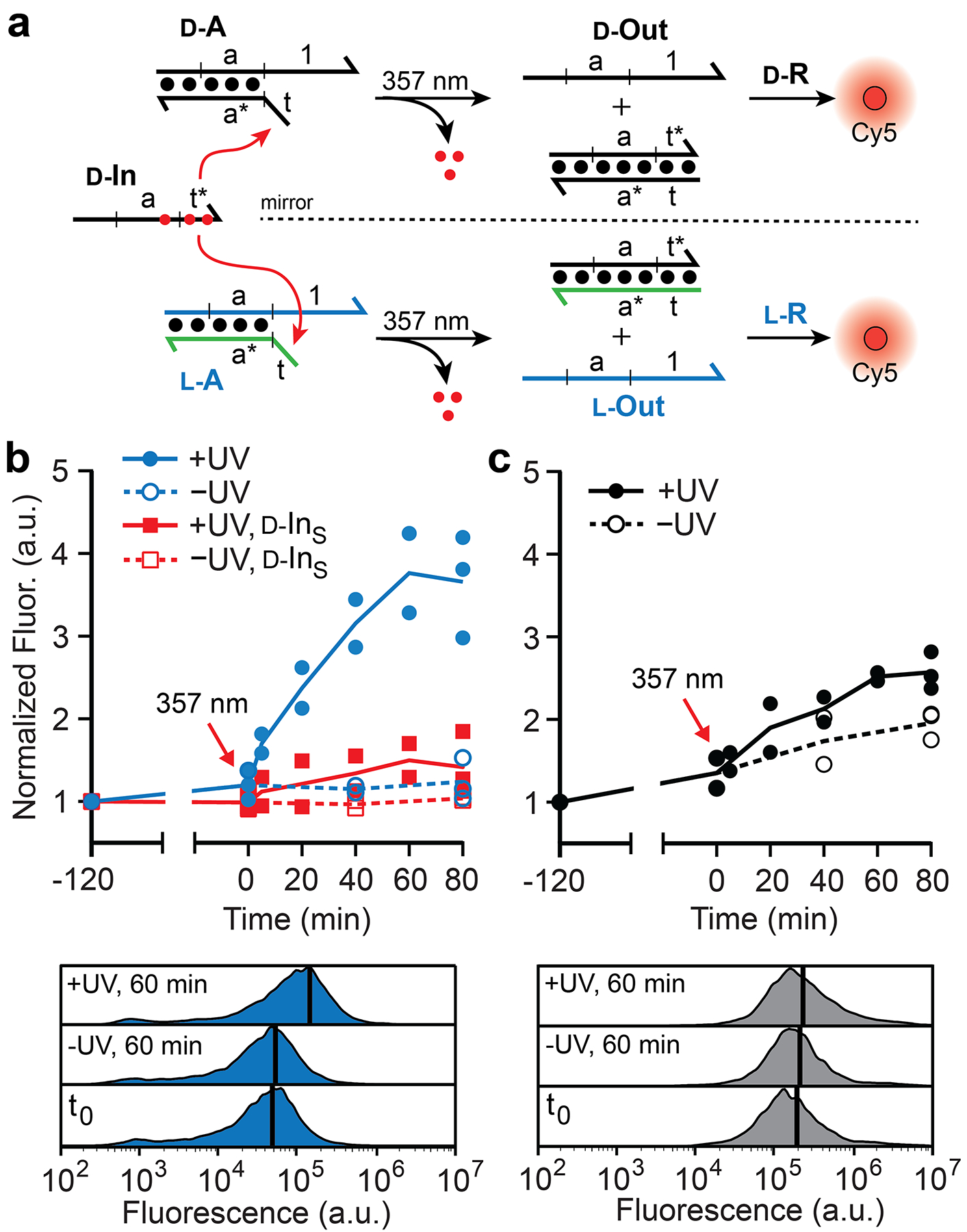
Comparison of d-DNA and l-DNA strand-displacement cascades in living cells. (a) Schematic illustration of the strand-displacement cascade. The achiral PNA strand is shown in green (Figure S1). The N-POM (6-nitropiperonyloxymethylene) caging group on thymidine nucleotides is represented by red dots. (b,c) Fluorescent monitoring of strand-displacement cascades in living cells by flow cytometry. Cells were transfected with either l-DNA (b) or d-DNA (c) versions of the reaction components depicted in panel (a) and photolyzed 2 hours later (where indicated). d-INS (red) indicates a scrambled input. All signals were normalized to the initial fluorescence measurement (−120 min). Individual data points from at least two independent experiments are plotted and connected by the mean. Representative histograms (below) display cell count versus fluorescence (black line indicates the mean value).
The proper operation of both d-DNA and l-DNA reaction cascades was first confirmed in a cell-free setting (Figure S6). We then packaged the input (d-IN) and reporter (d/l-R) components into liposomes separately from duplex d/l-A, and delivered both liposome complexes to cells simultaneously. Following a 2 hour pre-incubation that allowed for uptake, the cells were exposed to UV light (357 nm) for 3 minutes in order to uncage d-IN, and the reaction was monitored by flow cytometry and fluorescence microscopy (Figure 2b,c and Figure S7). We note that minimal cytotoxicity was observed following UV treatment (Figure S8). Upon photoactivation, the l-DNA reaction cascade proceeded rapidly leading to a ~4-fold enhancement in fluorescence relative to non-UV treated cells, which again showed no significant increase in signal (Figure 2b). Based on an unquenched positive control reaction, ~70% of l-reporter complexes were activated in response to UV treatment (Figure S9). This suggested that a majority of the reaction components were able to co-localize and react in cells despite being transfected separately. Importantly, an input strand with a scrambled toehold domain (d-INS) failed to activate an appreciable fluorescent signal upon photoactivation, confirming the heterochiral reaction cascade behaves as intended and has specificity for the correct input sequence. In contrast, the d-DNA reaction cascade behaved poorly in cells (Figure 2c). Transfection of the d-DNA circuit into cells resulted in a high background signal in the absence of UV, and only a modest fluorescent enhancement (< 1.5-fold) upon photoactivation of the input.
CONCLUSIONS
Taken together, our data clearly demonstrate that strand-displacement reaction systems constructed from l-DNA have superior intracellular stability and functionality compared to those constructed from the native polymer. To the best of our knowledge, this is the first time d-DNA and l-DNA strand-displacement has been directly compared in live cells. Most notably, l-DNA strand-displacement reactions show minimal “leak” despite prolonged exposure to the harsh intracellular environment and fast reaction kinetics upon activation, which together lead to dramatically improved signal-to-noise ratios compare to their d-DNA counterparts. Detailed understanding of the behavior of l-DNA and their complexes within living cells is essential for the broader adoption of the mirror-image approach in the field of DNA nanotechnology and beyond, and this study represents an important first step towards that goal. Importantly, by successfully demonstrating the operation of a multi-component heterochiral strand-displacement circuit in living cells, we have laid the foundation for interfacing endogenous d-nucleic acid signals (e.g. mRNAs) with robust l-DNA-based molecular circuits for practical, in vivo applications.
Supplementary Material
ACKNOWLEDGMENT
J.T.S is a CPRIT Scholar of Cancer Research supported by the Cancer Prevention and Research Institute of Texas (RR150038). Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R21EB027855.
Footnotes
Supporting Information
Materials and Methods; Nucleic acid sequences; representative flow cytometry and fluorescence microscopy data; nuclease stability assay; cell-free monitoring of strand-displacement cascades; cell viability assay.
The authors declare no competing financial interests.
REFERENCES
- (1).Simmel FC, Yurke B, and Singh HR (2019) Principles and applications of nucleic acid strand displacement reactions. Chem. Rev 119, 6326–6369. [DOI] [PubMed] [Google Scholar]
- (2).Zhang DY, and Seelig G (2011) Dynamic DNA nanotechnology using strand-displacement reactions. Nat. Chem 3, 103–113. [DOI] [PubMed] [Google Scholar]
- (3).Chen Y-J, Groves B, Muscat RA, and Seelig G (2015) DNA nanotechnology from the test tube to the cell. Nat. Nanotechnol 10, 748–760. [DOI] [PubMed] [Google Scholar]
- (4).Kabza AM, Young BE, and Sczepanski JT (2017) Heterochiral DNA strand-displacement circuits. J. Am. Chem. Soc 139, 17715–17718. [DOI] [PubMed] [Google Scholar]
- (5).Kabza AM, Young BE, Kundu N, and Sczepanski JT (2019) Heterochiral nucleic acid circuits. Emerg. Topics Life Sci 3, 501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Mallette TL, Stojanovic MN, Stefanovic D, and Lakin MR (2020) Robust heterochiral strand displacement using leakless translators. ACS Synth. Biol 9, 1907–1910. [DOI] [PubMed] [Google Scholar]
- (7).Young BE, Kundu N, and Sczepanski JT (2019) Mirror-image oligonucleotides: History and emerging applications. Chem. Euro. J 25, 7981–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Garbesi A, Capobianco ML, Colonna FP, Tondelli L, Arcamone F, Manzini G, Hilbers CW, Aelen JME, and Blommers MJJ (1993) L-DNAs as potential antimessenger oligonucleotides: a reassessment. Nucleic Acids Res. 21, 4159–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Hoehlig K, Bethge L, and Klussmann S (2015) Stereospecificity of oligonucleotide interactions revisited: No evidence for heterochiral hybridization and ribozyme/DNAzyme activity. PLoS ONE DOI: 10.1371/journal.pone.0115328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Zhong W, and Sczepanski JT (2019) A mirror image fluorogenic aptamer sensor for live-cell imaging of microRNAs. ACS Sens. 4, 566–570. [DOI] [PubMed] [Google Scholar]
- (11).Ke G, Wang C, Ge Y, Zheng N, Zhu Z, and Yang CJ (2012) L-DNA molecular beacon: a safe, stable, and accurate intracellular nano-thermometer for temperature sensing in living cells. J. Am. Chem. Soc 134, 18908–18911. [DOI] [PubMed] [Google Scholar]
- (12).Cui L, Peng R, Fu T, Zhang X, Wu C, Chen H, Liang H, Yang CJ, and Tan W (2016) Biostable L-DNAzyme for sensing of metal ions in biological systems. Anal. Chem 88, 1850–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Liang H, Xie S, Cui L, Wu C, and Zhang X (2016) Designing a biostable L-DNAzyme for lead(II) ion detection in practical samples. Anal. Methods 8, 7260–7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Groves B, Chen Y-J, Zurla C, Pochekailov S, Kirschman JL, Santangelo PJ, and Seelig G (2016) Computing in mammalian cells with nucleic acid strand exchange. Nat. Nanotechnol 11, 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Prokup A, Hemphill J, and Deiters A (2012) DNA computation: A photochemically controlled AND gate. J. Am. Chem. Soc 134, 3810–3815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


