Abstract
Frontotemporal dementia (FTD) refers to a complex spectrum of clinically and genetically heterogeneous disorders. Although fully penetrant mutations in several genes have been identified and can explain the pathogenic mechanisms underlying a great portion of the Mendelian forms of the disease, still a significant number of families and sporadic cases remains genetically unsolved. We performed whole exome sequencing in 100 patients with a late-onset and heterogeneous FTD-like clinical phenotype from Apulia and screened mendelian dementia and neuronal ceroid lipofuscinosis genes. We identified a nonsense mutation in SORL1 VPS domain (p.R744X), in 2 siblings displaying AD with severe language problems and primary progressive aphasia and a near splice-site mutation in CLCN6 (p.S116P) segregating with an heterogeneous phenotype, ranging from behavioural FTD to FTD with memory onset and to the logopenic variant of primary progressive aphasia in one family. Moreover 2 sporadic cases with behavioural FTD carried heterozygous mutations in the CSF1R Tyrosin kinase flanking regions (p.E573K and p.R549H). By contrast, only a minority of patients carried pathogenic C9orf72 repeat expansions (1%) and likely moderately pathogenic variants in GRN (p.C105Y, p.C389fs and p.C139R) (3%). In concert with recent studies, our findings support a common pathogenic mechanisms between FTD and neuronal ceroid lipofuscinosis and suggests that neuronal ceroid lipofuscinosis genes should be investigated also in dementia patients with predominant frontal symptoms and language impairments.
Subject terms: Clinical genetics, Mutation, Population genetics, Sequencing
Introduction
Frontotemporal dementia (FTD) refers to a clinical spectrum of disorders that are genetically, clinically, and neuropathologically heterogeneous. FTD is the second leading cause of early-onset dementia, after Alzheimer’s disease (AD)1. Genetics plays a pivotal role in the aetiology of FTD. 40–50% of FTD patients report a positive family history for disease2. Mutations in granulin (GRN) and microtubule-associated tau (MAPT) most typically cause early-onset (< 55 years) apparently Mendelian FTD. Hexanucleotide repeat expansions in the non-coding region of chromosome 9 open reading frame (C9orf72) underlie approximately 10% of all cases of FTD. Less frequently, mutations in the genes encoding TAR DNA-binding protein 43 (TDP-43), valosin containing protein (VCP), and the charged multivesicular body protein 2B (CHMP2B), Ubiquilin 2 (UBQLN2), prion protein (PRNP) and Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) have been reported3–6 and 16 pathogenic mutations in these genes have been detected in Italian FTD patients and explain part of the disease heritability (Table S1, Fig. 1).
Figure 1.
(A, B) Map of the genetic mutations in known FTD genes detected in Italy. Red circles describe the topographic area of the respective mutation. Blue circles display the area of provenience of the cohort investigated in the study, which has been shown in detail in Fig. 2B. Figure was generated using Power Point (https://www.microsoft.com/de-de/microsoft-365/powerpoint).
Nevertheless, a large fraction of FTD families and apparently sporadic cases with late-onset disease does not carry mutations in these genes. On the other hand, a growing body of evidence pointed to likely shared pathogenic mechanisms between frontal dementing syndromes with extrapyramidal signs and neuronal ceroid lipofuscinosis (NCL)7–10. It is likely that very rare, Mendelian, coding and private mutations may explain some of the remaining genetic component of FTD. Given the rare frequency of the pathogenic variants and the lack of large multigenerational pedigrees, GWA and linkage studies are unlikely to effectively detect such variants. By contrast, whole exome sequencing (WES) is a powerful tool to investigate the genetic landscape underlying complex syndromes11–13. Thus, we have applied WES to screen Mendelian dementia and neuronal ceroid lipofuscinosis genes in 100 familial and apparently sporadic patients from 6 small and isolated towns and villages in Apulia displaying a heterogeneous FTD-like phenotype.
Materials and methods
Cohort
One hundred clinically diagnosed FTD patients (47% female; 16% familial, 84% sporadic) were recruited in 6 small and isolated towns and rural villages on the Adriatic Sea cost of Apulia, an Italian southern region characterized by a distinctive historic and geographic isolation over the centuries: Bari (320.000 inhabitants, 116 km2), Andria (99.671 inhabitants, 407 km2), Lecce (95.269 inhabitants, 238 km2), Putignano (26.000 inhabitants, 99,11 km2), Polignano a mare (17.925 inhabitants, 62 km2), Tricase (17.421 inhabitants, 43,64 km2) (Fig. 1). Given their isolation, these areas display a high level of consanguinity and parental isonomy14 and the local population is highly inbred and enriched for rare causative alleles or highly penetrant risk factors of strong effect size. The patients were selected from the SLAP-DEM registry for rare neurodegenerative disorders in Puglia, south Italy. 75% displayed behavioural FTD (bvFTD), 21% primary progressive aphasia (PPA), and 1% patient showed FTD-ALS. Three patients were diagnosed with AD, and 2 of these were then diagnosed with FTD with memory onset and PPA during the disease progression. Average age at onset was 63 years (43–85y), 55% of the patients presented early-onset (< 65 years) and 24% very early-onset (≤ 55 years) (Table 1). All patients were evaluated with a complete neuropsychological assessment, structural and functional neuroimaging, blood chemistry tests and electromyography if the neurological examination showed motor-neuronal signs.
Table 1.
Cohort description.
| Patients | Female (%) | AAO | bvFTD | bvFTD_ALS | PPA | AD | FTD with memory onset | Parkinsonism/extrapyramidal signs | Pyramidal signs | Diffuse atrophy | Frontotemporal atrophy | Frontal/frontotemporal hypoperfusion | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Familial | 16 | 9 (56.2) | 64.8 (49–73) | 11 | 0 | 2 | 1 | 2 | 0 | 5 | 6 | 9 | 2 |
| Sporadic | 84 | 38 (45.2) | 62.1 (43–79) | 66 | 1 | 17 | 0 | 0 | 6 | 0 | 7 | 22 | 8 |
| Total | 100 | 47 (47) | 77 | 1 | 19 | 1 | 2 | 6 | 5 | 13 | 31 | 10 |
AAO age at onset, bvFTD behavioural frontotemporal dementia, ALS amyotrophic lateral sclerosis, PPA progressive primary aphasia.
This study and all experimental protocols were approved by the ethics committee on human research of the University Hospital and Polyclinic of Bari and the ethics committee of the Hospital of Lecce. Written informed consent was obtained from each subject enrolled in the study.
DNA extraction
DNA was extracted from blood using the automated DNA extractor AutoGenFlex STAR (AutoGen, Holliston, MA, USA) according to the manufacturer’s protocol.
C9orf72 repeat expansion study
A repeat-primed PCR was performed to screen for the presence of the GGGGCC hexanucleotide repeat expansion in C9orf72 as previously described15. Positive and negative controls were added to the polymerase chain reaction plate to assure accurate repeat analysis. Fragment length analysis was performed on an ABI 3730xl genetic analyzer (Applied Biosystems, Foster City, CA, USA) and data were analyzed using GeneScan software (version 4, ABI).
Exome sequencing
In an attempt to rapidly identify the underlying genetic mutation/s, we performed whole-exome sequencing on the DNA of each of the 16 affected individuals belonging to different families and 84 apparently sporadic FTD patients. Whole-exome sequencing (WES) was performed using the Extended Nextera Rapid-Capture Exome kit (Illumina, San Diego, CA, USA) and the Illumina HiSeq 2000 System (Illumina, San Diego, CA, USA). Quality control (QC), alignment, preprocessing and subsequent variant discovery were performed in accordance with the genome analysis toolkit (GATK) best practices16. A mean QC score for each sample’s FASTQ sequence file by cycle was calculated to ensure the technical quality. Samples were aligned to hg19 reference genome with BWA17 and Picard (http://picard.sourceforge.net) calculated alignment metrics. During preprocessing, duplicate reads were marked and consequently ignored. Mappings around indels were locally realigned to correct mapping artifacts. Base quality scores were recalibrated to prepare reads for variant discovery. HaplotypeCaller18 was performed per sample variant discovery on prepared reads. Overall, more than 200 million sequencing reads were produced for each sample, covering more than 12 billion bases. Approximately 98% of these were aligned to the human reference genome (hg19). On average, 92% of exome capture baits had at least 10 × depth and 87% at least 30 × depth.
The cohort was then jointly genotyped to capture the complete set of variants across all samples. Variant recalibration assigned a quality score commensurate to the probability of a SNP being a true variant. Then PLINK19 probed the heterozygosity, missingness and sex status of each sample. KING20 was used to measure pairwise relatedness between the subjects.
We used exome sequencing data to identify common (minor allele frequency [MAF] > 3%), rare (MAF < 3%), and very rare (MAF < 1%) coding variants in 26 genes causative for dementia (GRN [NM_002087], MAPT [NM_001123066], VCP [NM_007126], C9orf72 [NM_001256054], TREM2 [NM_001271821], TYROBP [NM_003332], UBQLN2 [NM_013444], PRNP [NM_000311], APP [NM_000484], PSEN1 [NM_000021], PSEN2 [NM_000447], SORL1 [NM_003105], CSF1R [NM_001288705], NOTCH3 [NM_000435], SNCA [NM_001146055], GBA [NM_001171811] or Neuronal Ceroid Lipofuscinoses: CLN10/CTSD [NM_001909], CLN1/PPT1 [NM_000391], CLN3 [NM_001286105], CLN5 [NM_006493], CLN6 [NM_017882], CLN7/MFSD8 [NM_152778], CLN4 [NM_017882], CLCN6 [NM_001256959], CLCN7 [NM_001256959] and SGSH [NM_000199]. The coding variants detected in these genes have been collected and analysed (Table 2). The pedigrees of the families were drawn with Progeny (http://www.progenygenetics.com/).
Table 2.
Coding mutations detected in the FTD-like cohort in Mendelian dementia and neuronal ceroid lipofuscinosis genes.
| Gene | Pathaway | Position | Rs ID | cDNA | Aa change | Domain | ExAc | CADD | FTD carriers (tot = 100) (%) | CTRLS Carrier HEX (tot = 368) | Phenotype | AAO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C9orf72 | Dementia | bvFTD | 44 | |||||||||
| GRN | Dementia | chr17:42,427,084 | Novel | c.G314A | p.C105Y | no | 26.5 |
Bari_RA_bvFTD_48 Bari_RMA_bvFTD_51 (2%) |
0 |
bvFTD bvFTD |
57 60 |
|
| GRN | Dementia | chr17:42,429,149 | Novel | c.1165delT | p.C389fs | no | NA | RSA_bvFTD_50 (1%) | 0 | bvFTD | 62 | |
| GRN | Dementia | chr17 :42,427,661 | Reported | c.T415C | p.C139R | no | 24 | RSA_bvFTD_50 (1%) | ||||
| MAPT | Dementia | chr17: 44,067,289 | Reported | c.C1228T | p.L410F | no | 25.2 | CM_bvFTD_60 (1%) | 0 | bvFTD | 55 | |
| VCP | Dementia | chr9:35,062,983- 35,062,985 | Novel | c.801_803del | p.267_268del | No | NA |
Bari_CA_bvFTD_48 PPA_Mpd_01_42 (2%) |
0 |
bvFTD PPA |
59 64 |
|
| TREM2 | Dementia | chr6:41,126,423 | Reported | c.C578A | p.P193Q | No | 5.951 | Bari_DP_PPA_35 (1%) | 0 | PPA | 78 | |
| PRNP | Dementia | chr20:4,680,089- 4,680,112 | Novel | c.223_246del | p.75_82del | No | NA | PPA_03_44 (1%) | 0 | PPA | 66 | |
| PSEN2 | Dementia | chr1:227,083,266 | Novel | c.C1333G | p.Q445E | No | 23.9 | bvFTD_19_35 (1%) | 0 | bvFTD | 69 | |
| SORL1 | Dementia | chr11:121,421,343 | Reported | c.C2230T | p.R744X | VPS10 | No | 38 |
H_II_2 H_II_4 (2%) |
0 | AD with severe language impairment/PPA |
62 69 |
| SORL1 | Dementia | chr11:121,495,891 | Reported | c.G6269T | p.G2090V | No | 28.4 | PPA_04_51 (1%) | 0 | PPA | 55 | |
| SORL1 | Dementia | chr11:121,440,881 | NA | c.G3239A | p.R1080H |
Benign 0.0116 |
23.1 | FTD_AOS_01_59 (1%) | 0 | bvFTD | 49 | |
| CSF1R | Dementia | chr5:149,441,322 | rs376280561 | c.G1717A | p.E573K | TK flanking region |
0.0% Probably-dam |
23.3 | Bari_DFC_bvFTD_35 (1%) | 0 | bvFTD | 78 |
| CSF1R | Dementia | chr5:149,441,393 | Reported | c.G1646A | p.R549H | TK flanking region | No | 23.0 | bvFTD_01_42 (1%) | 0 | bvFTD | 64 |
| CTSD | NCL | chr11:1,775,073 | Reported | c.G1031A | p.G344D | No | 22.6 | bvFTD_10_38 (1%) | 0 | bvFTD | 67 | |
| PPT1 | NCL | chr1:40,555,177 | Reported | c.132delT | p.F44fs | No | NA | bvFTD_03_34 (1%) | 0 | bvFTD | 86 | |
| CLCN6 | NCL | chr1:11,879,611 | Reported | c.T346C | p.S116P |
0.0233 Probably-dam |
23.3 |
E_II_1 E_II_2 E_II_5 (3%) |
0 |
FTD with memory onset PPA bvFTD |
71 68 75 |
|
| SGSH | NCL | chr17:78,184,307 | Reported | c.G1453A | p.G485S | No | 23.5 |
Bari_SA_bvFTD_57 RSA_bvFTD_50 |
0 |
bvFTD bvFTD |
56 62 |
Aa amino-acid, CTRLS controls from HEX database1, AAO age-at onset.
Variant filtering
All detected variants were functionally annotated with ANNOVAR21 and KGGSeq22. Variants were filtered for (1) heterozygous non synonymous, stop gain/loss, frameshift insertions/deletions and splice mutations that were (2) absent or very rare (minor allele frequency ≤ 0.001) in the public databases NHLBI ESP6500 (http://evs.gs.washington.edu/EVS/ ) and ExAC03 (http://exac.broadinstitute.org/) and (3) predicted pathogenic by at least one of the following in silico software algorithms: MetaSVM, MetaLR23 and CADD Phred score ≥ 20 (University of Washington and HudsonAlpha Institute for Biotechnology, Huntsville, AL).
Sanger sequencing
To verify that the variants reported in this study were not an artifact of the exome sequencing process, Sanger sequencing was performed using an ABI BigDye Terminator Cycle Sequencing Kit on an ABI 3730xl Sequencer. Sequence traces were analyzed using Sequencher (version 4.2; Gene Codes Corporation, Ann Arbor, MI, USA).
The pipeline of our study has been described in Fig. S1.
All methods were carried out in accordance with relevant guidelines and regulations.
Results
We identified 17 rare coding variants in the selected genes. Most of them, 12/17 (70%), were singletons, 5 were novel variants. In our cohort TYROBP, UBQLN2, APP, PSEN1, NOTCH3, SNCA, GBA, CLN2, CLN3, CLN5 did not present any rare coding variant (Table 2).
Dementia genes
Variants in GRN were detected in three subjects (3%). Two carried the same mutation (c.G314A, p.C105Y), previously shown to affect both the secretion of PGRN in cultured cells and the elastase cleavage of PGRN into GRN24. One patient (RSA_bvFTD_50) referred as apparently sporadic, carried two different variants in GRN. One missense mutation (c.T415C, p.C139R) in exon 5 leading to a predicted partial loss of functional protein and suggested as pathogenic by in silico and in vitro studies25. This mutation has been associated with behavioral frontotemporal dementia, semantic dementia, Alzheimer’s disease and corticobasal syndrome26. The second variant is a novel (e.g. not present in public databases) nucleotide deletion (c.1165delT, p.C389fs) in exon 10 predicted to give rise to a frameshift leading to the partial loss of function (Table 2). This patient presented with behavioral symptoms at age 63 (apathy, social retire and delusions). Four years later, he was completely socially inappropriate, unable to communicate and dependent in all daily activities with sphincter incontinence.
Only one individual (1%) carried a pathologic C9orf72 hexanucleotide repeat expansion (37 repeats). The carrier was a male sporadic case and displayed bvFTD with non-fluent aphasia and a very early age at onset (44 years).
We report a rare and likely non-pathogenic variant in PSEN2 p.Q445E, mapping outside the alpha helix surface of the transmembrane domains (TMs), where all the pathogenic mutations have been reported (alpha-helix rule)27.
Interestingly, we detected also 2 variants in CSF1R TK flanking regions (aa 538–581 and 911–972) (p.E573K and p.R549H). Although mutations in the TK domain (exons 12–22, aa 582–910) have been reported as pathogenic12, mutations in the TK flanking regions have been linked to AD28 and particularly p.E573K is characterized by a significantly decreased autophosphorylation compared to the wild-type CSF1R and has been previously reported in a patient presenting ischemic embolic stroke without the classical HDLS clinical feature but periventricular white matter abnormalities, unrelated to the recent infarct29.
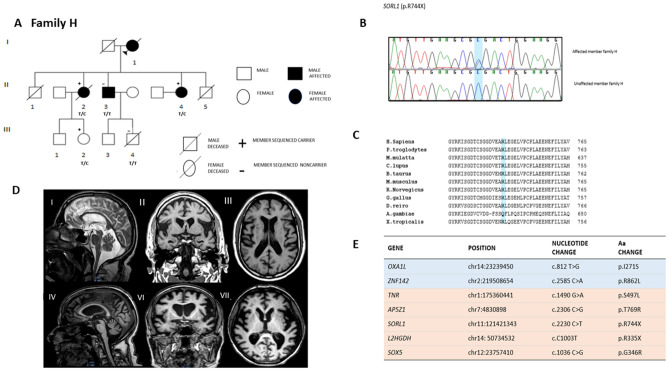
Moreover, we report one SORL1 mutation in the valosin-containing protein (VCP) domain (p.R744X) that was associated to AD with severe language impairment and PPA and was not detected in a member of the same family that had been initially diagnosed with AD and successively with FTD with memory onset (Table 3, Fig. 2A, B). SORL1 p.R744X was also found in an unaffected family member from the third generation (HIII2), aged 42 years, who should be considered at risk (average age at onset in Family H is 68 years).
Table 3.
Family H clinical features.
| Family member | Genetic screening | Gender | AAO | AAD | Duration | First symptom | Memory deficit | Behavioral problem | Language problems | Neurologic evaluation | CT/MRI | Clinical diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HI1 | Not performed | F | 60y | 65y | 5y | Behavioral, personality and mood changes | NA | Behavioral, personality and mood changes | NA | NA | NA | Clinical history was referred by relatives. Probable FTD-like syndrome |
| HII1 | Not performed | M | NA | 70y, lung cancer | NA | NA | NA | NA | NA | NA | NA | NA |
| HII2 | Negative | F | 62y | 80y | 18y | Memory problems and disorientation | Yes | None known | Paraphasic errors, agrammatism, mutism | Spastic hypertony, increased DTR, aphasia, global cognitive impairment | TC: diffuse cerebral atrophy | AD with severe language impairment/PPA |
| HII3 | Negative | F | 73y | Alive | 5y | Short-term memory deficits, disorientation | At onset, short-term memory impairment. Later, long-term memory problems, attention deficit | None known | None known | Right superior limb with II motoneuron signs. EMG negative for any pathological sign | MRI: predominant anterior atrophy | FTD with memory onset |
| HII4 | Negative | F | 69y | Alive | 6y | Language problems | None known | None known | Alexia, agraphia, perseverative language, mutism | Motor aphasia |
MRI: predominant anterior atrophy SPECT: left temporoparietal hypoperfusion |
PPA |
| HII5 | Not performed | M | NA | 60y, myocardial infarction | NA | NA | NA | NA | NA | NA | NA | NA |
| HIII5 | Not performed | M | NA | 13y, road traffic accident | NA | NA | NA | NA | NA | NA | NA | NA |
AAO age at onset, AAD age at death, AD Alzheimer’s disease, PPA primary progressive aphasia, bvFTD behavioral frontotemporal dementia, MRI magnetic resonance imaging, SPECT single photon emission computed tomography, DTR deep tendon reflex, EMG electromyography, Y years, NA not available, F female, M male.
Figure 2.
(A) Family H Pedigree. (B) SORL1 p.R744X Sanger sequencing chromatogram in an affected family member and in a control. (C) Conservation of SORL1 p.R744X across different species. (D) Brain MRI scans of 2 members of family H (HII3 and HII4). Sagittal (I, IV), coronal (II, VI), and axial (III, VII) T1-weighted images. (E) List of nonsynonymous coding variants segregating or detected in some of the affected members within Family H. In light blue are the variants which meet all the filter criteria: (1) novel variants; (2) segregating with the disease; (3) predicted as damaging by at least 2 out of 3 in silico prediction softwares (MUTATION TASTER, POLYPHEN2, SIFT) and 4) highly expressed in the brain and highly conserved (Grantham > 50, PhastCons > 0.4 and GERP > 4). In orange, variants which not fully segregate with the disease but may contribute to the disease phenotype. The pedigree of family H was drawn with Progeny (http://www.progenygenetics.com/).
The novel stop-gain mutation in SORL1 (p.R744X) clusters in a very well conserved domain across different species (Fig. 2C) and maps to exon 16, carrying several mutations that have been linked to familial and sporadic AD30–32. None of the sporadic FTD cases carried SORL1 variants in the VCP domain.
While a second heterozygous LoF mutation was identified within L2HGDH (p.R335X) in family H, this nonsense mutation did not segregate with disease. Finally, we report 4 mutations that cluster within genes highly expressed in the brain and already associated to developemental cognitive impairment and intellectual disability (TNR [p.S497L], AP5Z1[p.T769R], SOX5 [p.G346R], ZNF142 [p.R862L])33 (https://www.omim.org/) (Table S3, Fig. 2E). Moreover, OXA1L has been linked to mitochondrial encephalopathy and AP5Z1 and SOX5 to hereditary spastic paraplegia and ALS, respectively (https://www.omim.org/) 34. Although these mutations do not meet all the filter criteria, given the critical role in CNS development, they may be disease modifiers.
It is possible that these mutations (CLCN6 p.S116P, SORL1 p.R744X, L2HGDH p.R335X) lead to haploinsufficiency due to a nonsense-mediated decay (NMD) or either the generation of a truncated protein. Due to the lack of RNA samples available, it was not possible to perform a transcript analysis and demonstrate the absence of the mutant allele and therefore discriminate between the two mechanisms.
Family H
The clinical course of patients within Family H is characterized mainly by language impairment (HII2, HII4) and memory problems (HII2 and HII3) (Fig. 2A). The clinical diagnosis of affected family members includes probable AD, PPA and FTD with memory onset. The clinical features of the family members are summarized in Table 3.
The proband of the family died at 65 years of age and no samples were available for genetic evaluation. However, relatives described the patient as suffering from a dementing syndrome with behavioral and personality changes at the age of 60 years old.
HII2
At 62 years of age, the patient presented with memory impairment and spatiotemporal disorientation. Eight years after the onset of symptoms, she developed language problems that progressively worsened over four years with anomie, paraphasic errors and agrammatism progressing to mutism. At the age of 74 years, the patient was bed-ridden and completely dependent for all the daily activities. A neurological examination revealed spastic hypertony in all four limbs, increased and severe deep tendon reflex, mixed aphasia and global cognitive impairment. The clinical diagnosis was consistent with AD with severe language impairment. The patient deceased, aged 80 years old.
HII3
At 73 years of age, the patient developed short-term memory problems, depression and showed apathetic behavior. Three years later, aged 76 years, a neuropsychological examination revealed spatiotemporal disorientation. Long-term memory impairment and attention–execution deficits characterized the disease progression. An MRI scan, showed a marked anterior atrophy (Fig. 2D). The patient has been diagnosed with FTD with memory onset.
HII4
At 69 years of age, the patient presented with language impairment (anomie and stutter). Over the next four years, language problems progressed to complete mutism with alexia and agraphia. A neurological examination revealed a complete motor aphasia without any remarkable language comprehension impairment. Her behavior was socially appropriate. An MRI scan, performed three years after the onset of symptoms, revealed predominant anterior atrophy (Fig. 2D). A SPECT scan showed left temporo-parietal hypoperfusion. The patient was diagnosed with PPA.
Importantly, HII2 presented AD dementia and spastic paraplegia at the 4 limbs. Although this is a typical sign of patients with pathogenic mutations in PSEN135, we have not detected any coding mutation in PSEN1 in this family. However, we report a rare heterozygous missense mutation in AP5Z1, a gene that have been associated to autosomal recessive spastic paraplegia type 48 (SPG48)36. Nevertheless, the MRI did not present any typical feature of hereditary spastic tetraparesis: no periventricular white matter hyperintensities or thin corpus callosum (Fig. 2D). However, we cannot exclude that this mutation may modify the disease phenotype.
Neuronal ceroid lipofuscinosis genes
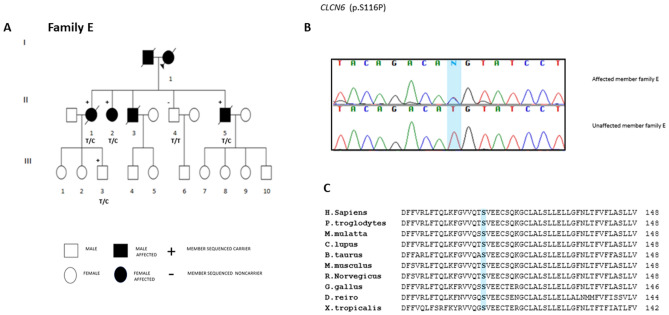
We report a novel and likely pathogenic variant identified in CLCN6 (p.S116P) leading to a T to C transition in the last nucleotide of exon 5 (c.346 in coding DNA reference sequence NM_001286.2), at position − 1 of the exon 5 splice donor site (Fig. 3B). The same mutation may alternatively result in exon 5 skipping or act as a missense mutation (c.T346C, predicting a p.S116P substitution), that may modify the protein activity.
Figure 3.
(A) Family E Pedigree. (B) CLCN6 p.S116P Sanger sequencing validation. (C) Conservation of CLCN6 p.S116P across different species. The pedigree of family E was drawn with Progeny (http://www.progenygenetics.com/).
This heterozygous mutation (CLCN6, p.S116P) segregates with the FTD-like phenotype in Family E and has been found in all the three affected siblings of Family E (EII1, EII2, EII5). Furthermore, also an asymptomatic member in the third generation (EIII3) carried the CLCN6 p.S116P variant. EIII3, aged 52 years, was likely too young to manifest the phenotype (average age at onset for the affected members was 71.3 years) (Table 4, Fig. 3A, B). Importantly, CLCN6 p.S116P was the only novel putative loss of function mutation predicted as damaging by at least 2 out of 3 in silico prediction softwares (MUTATION TASTER, POLYPHEN2, SIFT), highly expressed in the brain and highly conserved (Grantham > 50, PhastCons > 0.4 and GERP > 4) (Table S4, Fig. 3C), segregating with the disease phenotype in Family E, therefore this was the most likely mutation that could have explained the disease in this family.
Table 4.
Family E clinical features.
| Family member | Genetic screening | Gender | AAO | AAD | Duration | First symptom | Memory deficit | Behavioral problem | Language problems | Neurologic evaluation | CT/MRI | Clinical diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EI1 | Not performed | F | NA | 65y, pneumonia | NA | Referred cognitive problems | Referred memory impairment | None known | None known | NA | NA | Clinical history was referred by relatives. Probable dementing syndrome |
| EII1 | Negative | F | 71y | 80y, pneumonia | 9y | Short-term memory problems, Attention deficit | Short-term memory problems, Attention deficit | Mild personality changes, later in the disease | None known | Disoriented | NA | FTD with memory onset |
| EII2 | Negative | F | 68y | Alive | 6y | Language problems: paraphasic errors | None known | Apathy | Language problems: logopenia, anomia, paraphasic errors, mutism | None known |
MRI: moderate atrophy left frontal lobe, gliosis; SPECT: hypoperfusion left temporoparietal lobe |
PPA |
| EII3 | Not performed | M | Not known | 73y | NA | Cognitive impairment and behavioral problems | Referred memory impairment | Aggressivness | None known | NA | NA | Clinical history was referred by relatives. Probable dementing syndrome |
| EII5 | Negative | M | 75y | 84y, cardiac arrest | 9y | Behavioral problems, aggressivness | None known | Aggressiveness | Paraphasic errors, aphasia, mutism | NA | MRI: diffuse cortical atrophy, ( +) anterior frontotemporal lobes | bvFTD |
AAO age at onset, AAD age at death, AD Alzheimer’s disease, PPA primary progressive aphasia, bvFTD behavioral frontotemporal dementia, MRI magnetic resonance imaging, SPECT single photon emission computed tomography, DTR deep tendon reflex, EMG electromyography, Y years, NA not available, F female, M male.
Family E
Affected members of Family E display a heterogeneous clinical picture, ranging from probable AD, to bvFTD and PPA. The clinical features of the affected members are summarized in Table 4.
The proband of the family deceased at 65 years of age, due to pneumonia and could not be included in the genetic screening. However, relatives described the patient presenting with a dementing syndrome with cognitive and memory impairment.
EII1
At 71 years of age, the patient presented with deficits in short-term memory and attention. No behavioral changes or language impairment were reported. Mild personality fluctuations appeared only during the latter course of the disease. At 76 years of age, the patient was diagnosed with probable AD and later with FTD with memory onset. She was disoriented and died of pneumonia at 80 years of age.
EII2
Language impairment, progressively worsening with paraphasic errors, characterizes the onset of symptoms in patient EII2, aged 68 years. Two years later, at the age of 70 years, the patient was diagnosed with PPA; the disease gradually evolved to include apathy and mutism. A MRI scan revealed gliosis and modest atrophy accentuated in the left frontal lobe. A functional imaging using Technetium Tc 99 m single-photon emission computed tomography (SPECT) showed hypoperfusion particularly in the temporoparietal lobe, on the left hemisphere.
EII5
At the age of 75 years, the patient developed a change in personality with aggressive behavior. After three years, he displayed language problems worsening to include mutism and aphasia. After nine years of disease, the patient died due to a cardiac arrest. An MRI scan revealed diffuse cortical atrophy particularly marked in the anterior frontotemporal lobes.
Discussion
We carried out exome sequencing in 100 familial and apparently sporadic patients with FTD-like spectrum disorders and screened dementia and NCL genes.
Among the dementia genes, we identified 3 likely pathogenic variants in GRN in 3 sporadic cases (p.C105Y, p.C389fs, p.C139R), one C9orf72 expansion in one sporadic case, 2 CSF1R mutations in the TK flanking regions and one loss of function mutation in SORL1 (p.R744X) in 2/3 affected members of Family H. Additionally, we detected a novel putative LoF mutation in a NCL gene, CLCN6 p.S116P, segregating with FTD with memory onset and PPA in Family E (Table 2).
We recently reported a GRN novel splice site mutation, GRN c.709-2A > T, in a multigenerational family from the same geographic area37 in Apulia and in this cohort identified only 3 moderately to frankly pathogenic mutations in 3 apparently sporadic bvFTD cases and showed that GRN mutations may account for only a minority of FTD cases (6.4%), in contrast to the high prevalence of GRN mutations that have been described in a cohort of the nearby Calabria region, where the overall contribution of GRN mutations was 53% (17/32) increasing to 71.4% in patients with family history of dementia (15/21)38. Analogously, the frequency of C9orf72 expansions (1%) is much lower than the ones reported in other European countries and Italy particularly (6%)39 and this is likely not related to the North–South axis as the detected prevalence in Germany was 4.82% and, on the other hand, in Spain 25.49%39. This may further point to the isolation of these villages.
Interestingly, we reported 2 mutations in CSF1R in the TK domain flanking regions (aa 538–581 and 911–972): p.E573K and p.R549H, detected in 2 apparently sporadic patients with late-onset bvFTD. Although mutations in the TK regions of CSF1R (exons 12–22, aa 582–910) are causative for hereditary diffuse leukoencephalopathy with spheroids (HDLS), which clinically manifests as early-onset bvFTD-like with additional Parkinsonism, extrapyramidal or pyramidal signs40, also mutation in the CSF1R TK flanking regions have been already associated to early onset PPA28 and particularly p.E573K leads to a partial loss of the kinase activity and has been reported in a patient with ischemic embolic stroke without the typical clinical features of HDLS29, suggesting that missense mutations in the TK flanking regions leading to only a decreased TK activity may cause a milder phenotype compared to HDLS.
Among the dementia genes we detected a loss of function mutation in the VPS10 of SORL1 (Aa 124–757), p.R744X, in 2/3 affected members of Family H displaying late-onset AD with severe language impairment and PPA with pyramidal signs. This mutation was also found in an asymptomatic at risk member of the third generation (HIII2), aged 42 years (average age at onset in Family H is 68 years) and was not detected in another familial member, displaying FTD with memory onset, suggesting that SORL1 (p.R744X) may influence AD with language problems and PPA and that there may be additional genetic modifiers responsible for different phenotypic manifestations.
Importantly, SORL1 variants clustering in the VPS10 domain have been reported as pathogenic and to segregate within AD families41 particularly with extrapyramidal signs like parkinsonism42 and language impairment43 and also to vascular dementia44 and small vessel disease45. Therefore, our finding may support the role of SORL1 influencing motor function and language skills in dementing disorders.
Finally we report a near splice site mutation in CLCN6, p.S116P, segregating with an heterogeneous phenotype (bvFTD, FTD with memory onset and PPA) in Family E.
This mutation has been also reported in an asymptomatic member in the third generation (EIII3) that , aged 52 years, may manifest the phenotype later in life (average age at onset for the affected members was 71.3 years).
CLCN6 encodes for the protein CIC-6, a Cl- channel protein that is almost exclusively expressed in neurons. It co-localizes with late endosomes and mediates the exchange of endosomal Cl- for cytosolic H+46. It is plausible that this putative loss of function mutation may lead to a less efficient late endosomal acidification, thus compromising the protein degradation and the autophagosomal pathway, which are pH dependent. Therefore, it may affect TDP-43 degradation, contributing to its cytoplasmatic deposition.
Importantly, in vivo studies with Clcn6-/- mice recapitulate some of the histological and clinical features of late-onset NCL, characterized by the accumulation of storage material (saposinB, lamp-1, cathepsin D and lysosomal acid phosphatase) in the lysosomal system, leading to mild cognitive impairment and behavioral abnormalities46. Remarkably, a growing number of studies has shown that NCL and FTD may share common pathogenic mechanisms. First, GRN heterozygous LoF mutations cause FTD whereas homozygous LoF mutations cause NCL10,47.
Second, heterozygous mutations in the Cathepsin F (CTSF) gene, that in homozygosity are causative for adult-onset NCL, have been recently reported in a patient with early-onset FTD and motor symptoms9.
Third, NCL is characterized by pathological alterations typical of FTD and vice versa: NCL presents a different degree of TDP-43 phosphorylation and GRN-associated FTD is characterized by the elevation of lysosomal proteins and accumulation of saposin B, subunit c of mitochondrial ATP synthase (SCMAS), ubiquitin and p62 protein48. Fourth, TMEM106B, VCP, CHMP2B and SORT1, harbor variants identified as disease causing or risk factors for FTD and seem to play a role in endosomal trafficking49–52. As with CLCN6, TMEM106B and CHMP2B co-localize to the late endosomes and appear to be involved in the endosome-lysosome fusion. This represents a critical step for the autophagosome-mediated degradation of proteins and may be involved in TDP-43 turnover. Moreover, CLCN6 has been associated to increased levels of N-terminal cleavage product of the B-type natriuretic peptide (NT-proBNP), a well-established biomarker for dementia53,54.
The strength of our study relies on the enormous advantage of performing a genetic analysis in a very inbred FTD cohort from geographically and historically isolated areas and therefore enriched for rare alleles with high penetrance and strong effect size. On the other hand, a limitation of our study is represented by the lack of multigenerational and expanded families to analyze the segregation of rare pathogenic alleles.
Our study includes SORL1 VPS mutations, CSF1R and CLCN6 in the genetic spectrum associated to dementing syndromes with frontal signs, memory deficits, language impairment and pyramidal signs and in concert with a growing body of evidence supports the potential shared pathogenic ground underpinning FTD-like disorders and adult-onset neuronal ceroid-lipofuscinosis.
Supplementary information
Acknowledgements
This work was supported in part by the Intramural Research Programs of the National Institute on Aging and the National Institutes of Neurological Disorders and Stroke, within the National Institutes of Health, Department of Health and Human Services. Project number ZO1 AG000958.
Author contributions
A.S., G.L., C.S., R.C., M.H. planned the study, C.S., M.H., M.F., N.B. and T.P. performed the experiments, R.C. and G.L. performed the neurological examination, C.S., M.H., C.B., N.B., J.D., J.R.G. and A.S. performed the data analysis, C.S., R.C, M.H., A.S., G.L. drafted the manuscript, C.S., R.C., M.H., C.Z., M.F., C.B., N.B., J.D., J.R.G., T.P., A.S., G.L. revised the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-85494-x.
References
- 1.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/WNL.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 2.Rohrer JD, Warren JD. Phenotypic signatures of genetic frontotemporal dementia. Curr. Opin. Neurol. 2011;24:542–549. doi: 10.1097/WCO.0b013e32834cd442. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Rogaeva E. Motor neuron disease and frontotemporal dementia: sometimes related, sometimes not. Exp. Neurol. 2014;262(Pt B):75–83. doi: 10.1016/j.expneurol.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Gellera C, et al. Ubiquilin 2 mutations in Italian patients with amyotrophic lateral sclerosis and frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry. 2013;84:183–187. doi: 10.1136/jnnp-2012-303433. [DOI] [PubMed] [Google Scholar]
- 5.Giovagnoli AR, et al. Atypical frontotemporal dementia as a new clinical phenotype of Gerstmann–Straussler–Scheinker disease with the PrP-P102L mutation. Description of a previously unreported Italian family. Neurol. Sci. 2008;29:405–410. doi: 10.1007/s10072-008-1025-z. [DOI] [PubMed] [Google Scholar]
- 6.Le Ber I, et al. Homozygous TREM2 mutation in a family with atypical frontotemporal dementia. Neurobiol. Aging. 2014;35(2419):e23–2419.e25. doi: 10.1016/j.neurobiolaging.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward ME, et al. Individuals with progranulin haploinsufficiency exhibit features of neuronal ceroid lipofuscinosis. Sci. Transl. Med. 2017;9:eaah5642. doi: 10.1126/scitranslmed.aah5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arrant AE, Onyilo VC, Unger DE, Roberson ED. Progranulin gene therapy improves lysosomal dysfunction and microglial pathology associated with frontotemporal dementia and neuronal ceroid lipofuscinosis. J. Neurosci. 2018;38:2341–2358. doi: 10.1523/JNEUROSCI.3081-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Zee J, et al. Mutated CTSF in adult-onset neuronal ceroid lipofuscinosis and FTD. Neurol. Genet. 2016;2:e102. doi: 10.1212/NXG.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith KR, et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am. J. Hum. Genet. 2012;90:1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerreiro RJ, et al. Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia-like syndrome without bone involvement. JAMA Neurol. 2013;70:78–84. doi: 10.1001/jamaneurol.2013.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rademakers R, et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat. Genet. 2012;44:200–205. doi: 10.1038/ng.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, et al. Exome sequencing identifies mutations in ABCD1 and DACH2 in two brothers with a distinct phenotype. BMC Med. Genet. 2014;15:105. doi: 10.1186/s12881-014-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cizza G, et al. Clinical manifestations of highly prevalent corticosteroid-binding globulin mutations in a village in southern Italy. J. Clin. Endocrinol. Metab. 2011;96:E1684–1693. doi: 10.1210/jc.2011-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renton AE, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van der Auwera GA, et al. From FastQ data to high confidence variant calls: the genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013;43:11–10. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DePristo MA, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manichaikul A, et al. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26:2867–2873. doi: 10.1093/bioinformatics/btq559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucl. Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M-X, Gui H-S, Kwan JSH, Bao S-Y, Sham PC. A comprehensive framework for prioritizing variants in exome sequencing studies of Mendelian diseases. Nucl. Acids Res. 2012;40:e53. doi: 10.1093/nar/gkr1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong C, et al. Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum. Mol. Genet. 2015;24:2125–2137. doi: 10.1093/hmg/ddu733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karch CM, et al. Missense mutations in progranulin gene associated with frontotemporal lobar degeneration: study of pathogenetic features. Neurobiol. Aging. 2016;38(215):e1–215.e12. doi: 10.1016/j.neurobiolaging.2015.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gómez-Tortosa E, et al. Plasma progranulin levels in cortical dementia phenotypes with asymmetric perisylvian atrophy. Eur. J. Neurol. 2013;20:1319–1324. doi: 10.1111/ene.12211. [DOI] [PubMed] [Google Scholar]
- 26.Piaceri I, et al. Association of the variant Cys139Arg at GRN gene to the clinical spectrum of frontotemporal lobar degeneration. J. Alzheimers Dis. 2014;40:679–685. doi: 10.3233/JAD-132126. [DOI] [PubMed] [Google Scholar]
- 27.Hardy J, Crook R. Presenilin mutations line up along transmembrane alpha-helices. Neurosci. Lett. 2001;306:203–205. doi: 10.1016/S0304-3940(01)01910-3. [DOI] [PubMed] [Google Scholar]
- 28.Sassi C, et al. Mendelian adult-onset leukodystrophy genes in Alzheimer’s disease: critical influence of CSF1R and NOTCH3. Neurobiol. Aging. 2018;66(179):e17–179.e29. doi: 10.1016/j.neurobiolaging.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konno T, et al. Partial loss of function of colony-stimulating factor 1 receptor in a patient with white matter abnormalities. Eur. J. Neurol. 2018;25:875–881. doi: 10.1111/ene.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen OM, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc. Natl. Acad. Sci. U.S.A. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogaeva E, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat. Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pottier C, et al. High frequency of potentially pathogenic SORL1 mutations in autosomal dominant early-onset Alzheimer disease. Mol. Psychiatry. 2012;17:875–879. doi: 10.1038/mp.2012.15. [DOI] [PubMed] [Google Scholar]
- 33.Jones AR, et al. Stratified gene expression analysis identifies major amyotrophic lateral sclerosis genes. Neurobiol. Aging. 2015;36(2006):e1–9. doi: 10.1016/j.neurobiolaging.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Thompson K, et al. OXA1L mutations cause mitochondrial encephalopathy and a combined oxidative phosphorylation defect. EMBO Mol. Med. 2018;10:e9060. doi: 10.15252/emmm.201809060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlstrom H, et al. Variable phenotype of Alzheimer’s disease with spastic paraparesis. J. Neurochem. 2008;104:573–583. doi: 10.1111/j.1471-4159.2007.05038.x. [DOI] [PubMed] [Google Scholar]
- 36.Pensato V, et al. Overlapping phenotypes in complex spastic paraplegias SPG11, SPG15, SPG35 and SPG48. Brain. 2014;137:1907–1920. doi: 10.1093/brain/awu121. [DOI] [PubMed] [Google Scholar]
- 37.Sassi C, et al. A novel splice-acceptor site mutation in GRN (c.709–2 A>T) causes frontotemporal dementia spectrum in a large family from Southern Italy. J. Alzheimers Dis. 2016 doi: 10.3233/JAD-151170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernardi L, et al. Epidemiology and genetics of frontotemporal dementia: a door-to-door survey in southern Italy. Neurobiol. Aging. 2012;33(2948):e1–2948.e10. doi: 10.1016/j.neurobiolaging.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Zee J, et al. A pan-European study of the C9orf72 repeat associated with FTLD: geographic prevalence, genomic instability, and intermediate repeats. Hum. Mutat. 2013;34:363–373. doi: 10.1002/humu.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konno T, Kasanuki K, Ikeuchi T, Dickson DW, Wszolek ZK. CSF1R-related leukoencephalopathy: a major player in primary microgliopathies. Neurology. 2018;91:1092–1104. doi: 10.1212/WNL.0000000000006642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vardarajan BN, et al. Coding mutations in SORL1 and Alzheimer disease. Ann. Neurol. 2015;77:215–227. doi: 10.1002/ana.24305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuccaro ML, et al. SORL1 mutations in early- and late-onset Alzheimer disease. Neurol. Genet. 2016;2:e116. doi: 10.1212/NXG.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thonberg H, et al. Identification and description of three families with familial Alzheimer disease that segregate variants in the SORL1 gene. Acta Neuropathol. Commun. 2017;5:43. doi: 10.1186/s40478-017-0441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuenco KT, et al. Association of distinct variants in SORL1 with cerebrovascular and neurodegenerative changes related to Alzheimer disease. Arch. Neurol. 2008;65:1640–1648. doi: 10.1001/archneur.65.12.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blumenau S, et al. Investigating APOE, APP-Aβ metabolism genes and Alzheimer’s disease GWAS hits in brain small vessel ischemic disease. Sci. Rep. 2020;10:7103. doi: 10.1038/s41598-020-63183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poët M, et al. Lysosomal storage disease upon disruption of the neuronal chloride transport protein ClC-6. Proc. Natl. Acad. Sci. U.S.A. 2006;103:13854–13859. doi: 10.1073/pnas.0606137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed Z, et al. Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am. J. Pathol. 2010;177:311–324. doi: 10.2353/ajpath.2010.090915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Götzl JK, et al. Common pathobiochemical hallmarks of progranulin-associated frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis. Acta Neuropathol. 2014;127:845–860. doi: 10.1007/s00401-014-1262-6. [DOI] [PubMed] [Google Scholar]
- 49.Brady OA, Zheng Y, Murphy K, Huang M, Hu F. The frontotemporal lobar degeneration risk factor, TMEM106B, regulates lysosomal morphology and function. Hum. Mol. Genet. 2013;22:685–695. doi: 10.1093/hmg/dds475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tresse E, et al. VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy. 2010;6:217–227. doi: 10.4161/auto.6.2.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urwin H, et al. Disruption of endocytic trafficking in frontotemporal dementia with CHMP2B mutations. Hum. Mol. Genet. 2010;19:2228–2238. doi: 10.1093/hmg/ddq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu F, et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron. 2010;68:654–667. doi: 10.1016/j.neuron.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Greco MF, et al. Genome-wide association analysis and fine mapping of NT-proBNP level provide novel insight into the role of the MTHFR-CLCN6-NPPA-NPPB gene cluster. Hum. Mol. Genet. 2011;20:1660–1671. doi: 10.1093/hmg/ddr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mirza SS, et al. The N-terminal pro B-type natriuretic peptide, and risk of dementia and cognitive decline: a 10-year follow-up study in the general population. J. Neurol. Neurosurg. Psychiatr. 2015 doi: 10.1136/jnnp-2014-309968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).