Abstract
Summary: A patient with a superior sagittal sinus thrombosis had progressively worsening symptoms and signs that resolved after IV heparin therapy. MR imaging revealed abnormalities in diffusion, similar to those seen with acute arterial stroke. Abnormalities shown on a T2-weighted fast spin-echo and fluid-attenuated inversion recovery images resolved completely. The findings in this report contradict those from previous reports that suggest diffusion-weighted imaging with quantitative apparent diffusion coefficients may be used in selecting patients for dural venous sinus thrombolysis.
Dural sinus thrombosis, combined with thrombosis of the deep or cortical cerebral venous system and resulting venous stroke, is more common than once thought. It occurs in patients of all age groups, although it mainly occurs in those aged 20–35 y. The mortality rate once was as high as 10–30% (1), but it is now approximately 10% or lower (2, 3) because of improved early detection, which is attributable to the availability of CT and MR imaging, to the addition of angiographic features to these techniques (2, 4), and to more adequate therapy (4). Several factors, including infancy (not the neonatal period [5]) and advanced age, rapid onset with coma and focal deficits, and thrombosis largely affecting the deep venous system or cerebellar veins, are associated with poor prognosis (3).
Heparin therapy can be used to achieve approximately 70% complete recovery (1). Heparin can stop the progress of thrombosis and save venous collaterals, and it sometimes allows the spontaneous thrombolytic process to achieve partial or complete recanalization (6). Patients whose condition deteriorates clinically while undergoing heparin therapy are known to benefit from aggressive microcatheter-directed dural venous sinus thrombolysis, usually with urokinase or other thrombolytic agents (2–4, 7). The use of mechanical thrombolysis, with a rheolytic catheter, can be reserved for patients who have poor responses to local infusions of urokinase, a rapid neurologic decline, or contraindications to thrombolytics (2).
In a recent report, it was suggested that diffusion-weighted imaging, with the use of quantitative apparent diffusion coefficients (ADCs), has potential application in triaging patients for IV heparin therapy and microcatheter-directed dural venous sinus thrombolysis (7). Low ADC values (< 0.20 × 10−3 mm2/s) were associated with poor outcome in this report, whereas an area with less severe reduction (> 0.30 × 10−3 mm2/s) did not develop a corresponding anatomic abnormality and reversed completely. These findings led to the assumption that ADC values greater than 0.30 × 10−3 mm2/s might be reversible and might benefit from dural venous sinus thrombolysis. However, our report shows that an equal reduction of ADC values (0.34–0.46 × 10−3 mm2/s) can resolve completely with the use of IV heparin without microcatheter-directed dural venous sinus thrombolysis, even in the presence of an anatomic abnormality.
Case Report
A 37-year-old woman was admitted to the hospital 4 d after the onset of a progressively worsening headache that was complicated by sudden paresis in the right arm, transient paresthesias in both arms and legs, and transient paresis in the left arm and left side of the face.
Results of nonenhanced CT of the brain were normal, and MR imaging was then performed. The following sequences were performed with a 1.5-T, whole-body MR imaging system by using high-performance gradients (peak strength, 25 mT/m) and a standard head coil: 1) an axial T2-weighted fast spin-echo sequence (5500/128/3 [TR/TE/excitations]; section thickness, 6 mm); 2) an axial fluid-attenuated inversion recovery (FLAIR) sequence (6000/105/2200; section thickness, 6 mm; flip angle, 180°); 3) an axial nonenhanced T1-weighted spin-echo sequence (550/14/3; section thickness, 6 mm); 4) diffusion-weighted imaging, including an axial echo-planar spin-echo sequence (800/123; section thickness, 6 mm; field of view, 250 × 250 cm; matrix, 128 × 200; flip angle, 90°; b values of 0, 300, and 1200 s/mm2; sensitizing gradient in the z direction) and calculated ADC maps; 5) an axial contrast-enhanced T1-weighted sequence; and 6) an oblique parasagittal 2D multisection fast low-angle snap shot MR venographic sequence (30/9/1; section thickness, 3 mm; matrix, 256 × 256; field of view, 25 × 25 cm; flip angle, 50°) with maximum intensity projection reconstruction.
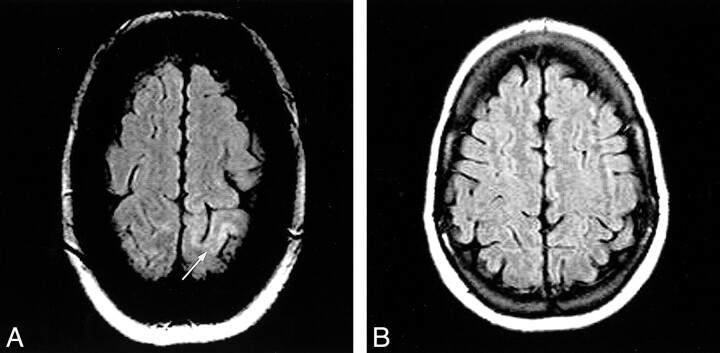
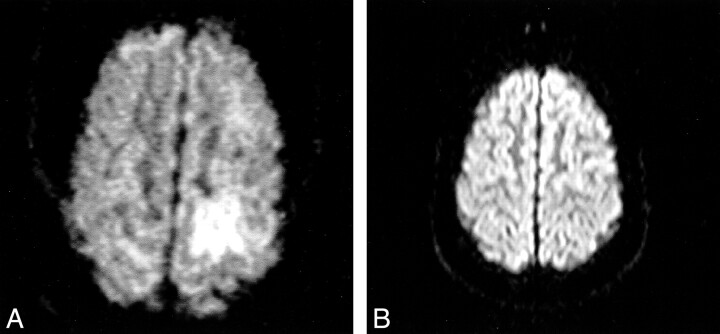
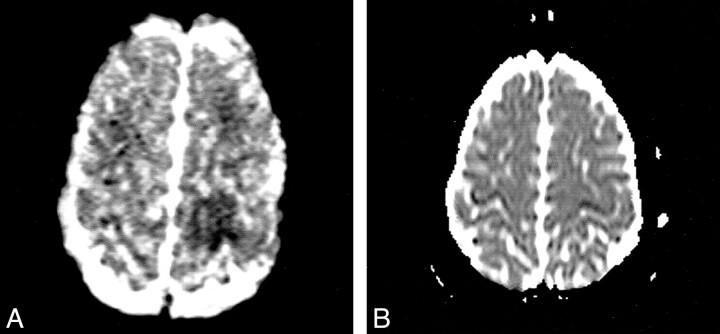
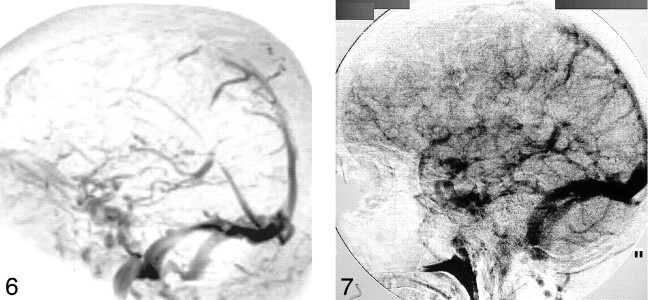
Moderate, mainly cortical hyperintensity on T2-weighted fast spin-echo (Fig 1) and FLAIR (Fig 2A) images was accompanied by an area of more pronounced hyperintensity on diffusion-weighted images (Fig 3A) and by a clear decrease in ADC values (Fig 4A), in the range of 0.34–0.46 × 10−3 mm2/s, compared with 0.68 × 10−3 mm2/s in the contralateral hemisphere. The contrast-enhanced T1-weighted spin-echo image (Fig 5) revealed abnormal cortical contrast enhancement, and thrombosis of the superior sagittal sinus was confirmed with MR venography (Fig 6) and conventional angiography (Fig 7).
fig 1.
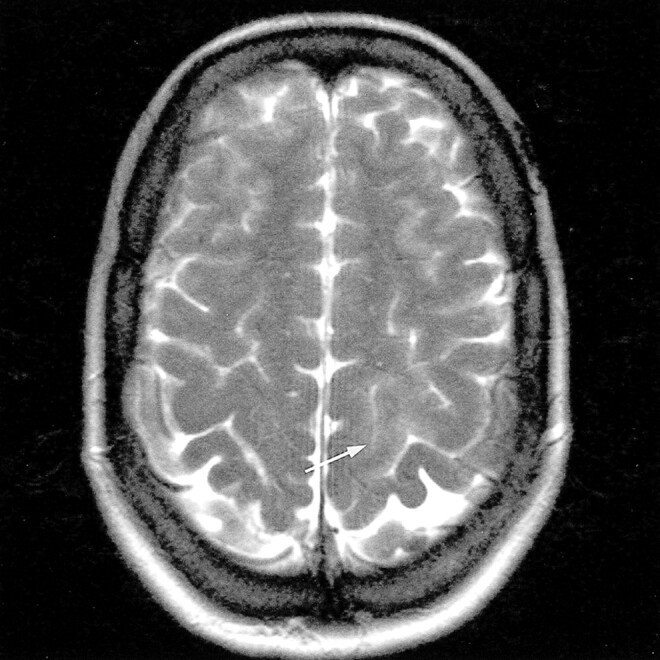
Axial T2-weighted fast spin-echo MR image (5500/128/3) shows a moderate, mainly cortical hyperintensity in the left posterior parietal lobe (arrow)
fig 2.
Axial FLAIR MR images (6000/105/2200).
A, Image shows a moderate, mainly cortical hyperintensity in the left posterior parietal lobe (arrow).
B, Follow-up image shows no abnormality.
fig 3.
Axial diffusion-weighted echo-planar MR images (800/123) obtained with a z-sensitizing gradient.
A, Image shows a more pronounced, mainly cortical hyperintensity in the left posterior parietal lobe.
B, Follow-up image shows no abnormalities.
fig 4.
Corresponding ADC maps.
A, Map shows a clear decrease in ADC values in the left posterior parietal lobe (hypointensity), compared with the contralateral side.
B, Follow-up map shows normalized ADC values in the left posterior parietal lobe, compared with the contralateral side.
fig 5.
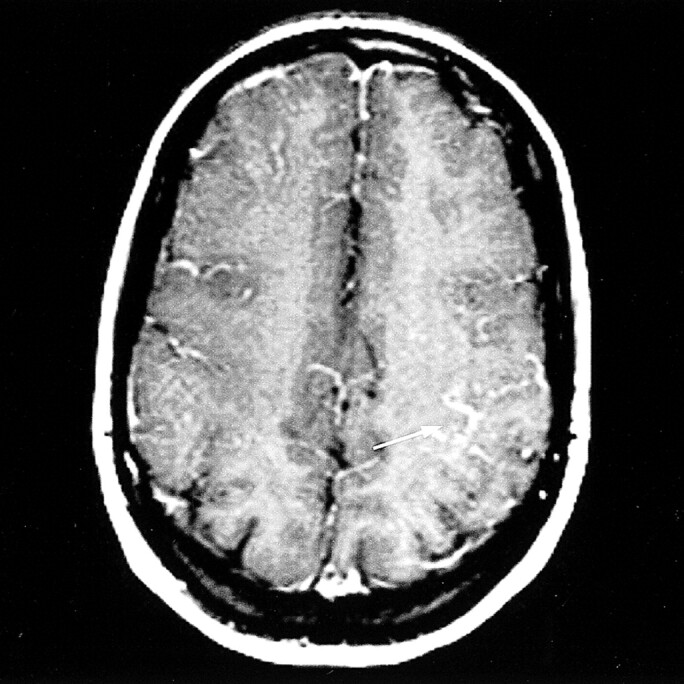
Axial contrast-enhanced T1-weighted spin-echo MR image shows abnormal cortical enhancement in the left posterior parietal lobe (arrow)
fig 6.
Maximum intensity projection reconstruction of an MR venogram shows thrombosis in the anterior half of the superior sagittal sinus.
fig 7. Conventional angiogram shows thrombosis in the anterior half of the superior sagittal sinus.
IV heparin therapy was started. Two weeks later, only discrete reduction of the fine-motor activity in the right hand remained, and this disappeared completely a few weeks later. Follow-up MR examination, including FLAIR (Fig 2B) and diffusion-weighted imaging and ADC mapping (Figs 3B and 4B), was performed, and no abnormalities were revealed. Oral anticoagulant therapy was continued for 6 mo.
The only possible etiologic factor for venous thrombosis that could be withheld was oral contraceptives, which is known to be the etiologic factor in 10% of cases (3).
Discussion
In 1998, Corvol et al (8) reported the findings in a case of extensive thrombosis of the superior sagittal sinus and left lateral sinus. There was a large frontoparietal hyperintensity on FLAIR images and only a discrete hyperintensity on diffusion-weighted images, with a very slight decrease in ADC values (0.53 × 10−3 mm2/s compared with 0.61 × 10−3 mm2/s in the contralateral hemisphere). These findings were explained as being the result of prominent vasogenic edema associated with mild cytotoxic edema. The patient was treated with IV heparin, with a favorable outcome.
In 1999, Keller et al (9) reported the findings in a case of deep cerebral venous thrombosis with extensive hyperintensities in the basal ganglia on T2-weighted images and hypointensities on diffusion-weighted images, with increased ADC values (1.1–1.6 × 10−3 mm2/s). These findings were explained as being the result of vasogenic edema. The patient was treated with IV heparin and had a total clinical recovery; no parenchymal defects were revealed during follow-up MR examinations.
Then in 2000, Manzione et al (7) reported the findings in a case of superior sagittal sinus thrombosis and right transverse sinus thrombosis. There was frontal hyperintensity on T2-weighted images and two more extensive hyperintensities on diffusion-weighted images associated with an area of severe (0.2 × 10−3 mm2/s) and moderate (0.3 × 10−3 mm2/s) reductions in ADC values. Despite IV heparin therapy, the patient's clinical state worsened, and intradural catheter-directed venous sinus thrombolysis was performed, with a favorable outcome. Interestingly, the lesion with a severe reduction in ADC values was associated with a small residual lesion at follow-up MR examination, while the area with a moderate reduction in ADC values reversed completely. This finding correlates with those in our report in which the reduction of ADC values was moderate (0.34–0.46 × 10−3 mm2/s). In our case, intradural sinus thrombolysis was not performed and IV heparin therapy was successful, resulting in the absence of a residual parenchymal lesion at follow-up MR examination.
These apparently divergent results raise a number of issues. First, we postulate that the pathogenesis of venous infarction differs considerably from the pathogenesis of arterial ischemic infarction. The initial event in venous infarction is the increase in venous pressure, which is associated with disruption of the capillary tight junctions; this increases the volume of extracellular water (vasogenic edema). These lesions are completely reversible, provided successful venous thrombolysis occurs, as reported in the aforementioned reports (8, 9).
Subsequently, an increase in intracellular water volume follows (cytotoxic edema), resulting in restriction of the diffusion of water and hyperintensity on diffusion-weighted images (10, 11). The mechanism might be energy failure, with loss of Na+/K+ pump activity, as in arterial stroke. However, in contrast to the lesions in arterial stroke, the “bright” lesions on diffusion-weighted images of venous infarction might be more amenable to complete recovery if successfully treated, as we report. We know from studies of acute human stroke, with xenon CT, that cerebral blood flow of 6 mL or less per 100 g per minute produces irreversible infarction, while the ischemic penumbra with flow values of 7–20 mL per 100 g per minute may be salvaged after restoration of normal flow (12). In venous infarction, hypoperfusion develops progressively, and we postulate that it probably seldom decreases below the threshold of 6 mL per 100 g per minute, more or less, because perfusion of the affected brain tissue might still be possible at lower flow rates if the blood is drained through collateral pathways (13). The swollen cells might be functionally but not irreversibly damaged; therefore, they have a potential for recovery (13). Also, in our case, the hyperintensity on diffusion-weighted images and the low ADC values, which suggested cytotoxic edema, were not associated with a poor outcome after IV heparin therapy, as previously suggested (7). Therefore, these findings may be insufficient to warrant starting aggressive dural venous sinus thrombolytic therapy, especially when the patient's clinical state is stable or improving with IV heparin therapy. Further data are required to evaluate the value of ADC quantification in this setting.
Footnotes
Address reprint requests to Els Y. Peeters, MD, Department of Radiology, University Hospital Vrije Universiteit Brussel, Laarbeeklaan 101, B-1090 Brussels, Belgium.
References
- 1.Greiner FG, Takhtani D. Neuroradiology case of the day. Radiographics 1999;19:1098-1101 [DOI] [PubMed] [Google Scholar]
- 2.Baker MD, Opatowsky MJ, Wilson JA, Glazier SS, Pearse Morris P. Rheolytic catheter and thrombolysis of dural venous sinus thrombosis: a case series. Neurosurgery 2001;48:487-494 [DOI] [PubMed] [Google Scholar]
- 3.Bousser MG. Cerebral venous thrombosis: diagnosis and management. J Neurol 2000;247:252-258 [DOI] [PubMed] [Google Scholar]
- 4.Philips MF, Bagley LJ, Sinson GP, et al. Endovascular thrombolysis for symptomatic cerebral venous thrombosis. J Neurosurg 1999;90:65-71 [DOI] [PubMed] [Google Scholar]
- 5.Lafitte F, Boukobza M, Guichard JP, Reizine D, Woimant F, Merland JJ. Deep cerebral venous thrombosis: imaging in eight cases. Neuroradiology 1999;41:410-418 [DOI] [PubMed] [Google Scholar]
- 6.Brucker AB, Vollert-Rogenhofer H, Wagner M, et al. Heparin treatment in acute cerebral sinus venous thrombosis: a retrospective clinical and MR analysis of 42 cases. Cerebrovascular Dis 1998;8:331-337 [DOI] [PubMed] [Google Scholar]
- 7.Manzione J, Newman GC, Shapiro A, Santo-Ocampo R. Diffusion- and perfusion-weighted MR imaging of dural sinus thrombosis. AJNR Am J Neuroradiol 2000;21:68-73 [PMC free article] [PubMed] [Google Scholar]
- 8.Corvol JC, Oppenheim C, Manaï R, et al. Diffusion-weighted magnetic resonance imaging in a case of cerebral venous thrombosis. Stroke 1998;29:2649-2652 [DOI] [PubMed] [Google Scholar]
- 9.Keller E, Flacke S, Urbach H, Schild HH. Diffusion- and perfusion-weighted magnetic resonance imaging in deep cerebral venous thrombosis. Stroke 1999;30:1144-1146 [DOI] [PubMed] [Google Scholar]
- 10.Le Bihan D. Molecular diffusion nuclear magnetic resonance imaging. Magn Reson Q 1991;7:1-30 [PubMed] [Google Scholar]
- 11.Pierpaoli C, Righini A, Libfante I, Tao-Cheng JH, Alger JR, Ci Chiro G. Histopathologic correlates of abnormal water diffusion in cerebral ischemia: diffusion-weighted MR imaging and light and electron microscopic study. Radiology 1993;189:439-444 [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann A, Firlik A, Fukui M, Wechsler L, Jungries C, Yonas H. Ischemic core and penumbra in human stroke. Stroke 1999;30:93-99 [DOI] [PubMed] [Google Scholar]
- 13.Ducreux D, Oppenheim C, Vandamme X, et al. Diffusion-weighted imaging patterns of brain damage associated with cerebral venous thrombosis. AJNR Am J Neuroradiol 2001;22:261-268 [PMC free article] [PubMed] [Google Scholar]