Abstract
Summary: We report MR findings in a case of a solitary fibrous tumor involving the buccal space in a middle-aged man. On MR images, most of the mass was isointense and hyperintense to the muscle on T1- and T2-weighted images, respectively, and showed homogeneously strong enhancement on contrast-enhanced T1-weighted images. The medial peripheral portion, which was isointense on T2-weighted images and showed less enhancement on contrast-enhanced T1-weighted images, corresponded to the hypocellular and collagenous sclerotic area on pathologic correlation.
Solitary fibrous tumor is a rare spindle-cell tumor that typically appears as a pleura-based mass despite the recent increasing recognition of its extrapleural location. In the English-language medical literature, only a few cases of solitary fibrous tumor of the buccal space have been described, and they have poor descriptions of the radiologic findings (1–3). We describe the MR findings correlated with pathologic findings of this rare tumor involving the buccal space.
Case Report
A 46-year-old man presented with a 1-mo history of a painless mass in the right cheek. The mass was hard and movable. He denied a history of trauma or surgery to the cheek and had no masticatory problem. A protruding mass was evident behind the parotid duct opening at the right buccal mucosa.
MR imaging at 1.5-T revealed a well-circumscribed solid mass measuring 2 × 2.3 × 2.7 cm in the right posterior buccal space, with anterior displacement of the parotid duct (Fig 1A). The mass abutted and compressed the anterior portion of the right masseter muscle; however, it was separated from the mandible and maxilla (Fig 1A–D). The mass showed homogeneous signal isointensity relative to that of muscle on T1-weighted images (Fig 1A), and mainly signal hyperintensity with isointensity in the medial peripheral portion on T2-weighted images (Fig 1B). The mass showed homogeneously strong enhancement; the less enhancing portion was the medial peripheral portion on contrast-enhanced fat-suppressed T1-weighted images (Fig 1C and D). CT was not performed, because additional information was not expected in this tumor.
fig 1.
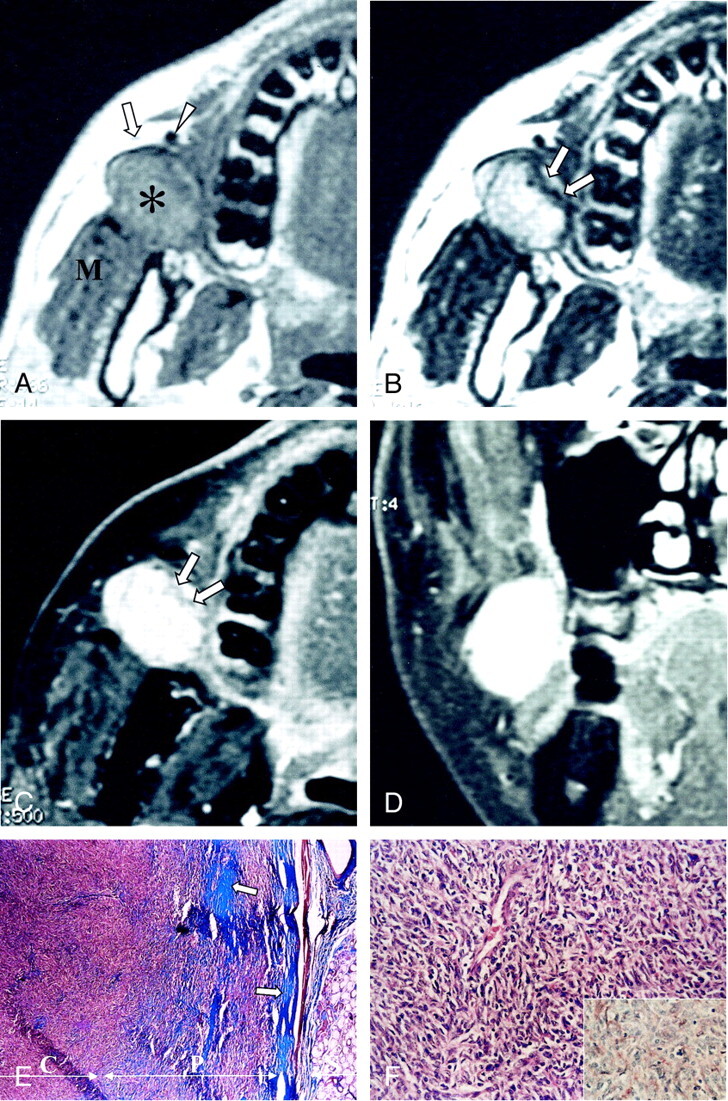
Images in a 46-year-old man with a painless right cheek mass.
A, Axial T1-weighed MR image (466/14 [TR/TE]) shows a well-marginated mass (asterisk) that is isointense to the muscle in the right posterior buccal space. The mass displaces the parotid duct (arrow) and the facial vein (arrowhead) anteriorly and compresses the masseter muscle (M) posteriorly.
B, Axial T2-weighted MR image (4016/104) shows that the mass has mainly high signal intensity, with linear signal isointensity in the medial peripheral portion (arrows).
C and D, Axial (C) and coronal (D) contrast-enhanced T1-weighted MR images (500/14) reveal homogeneously strong enhancement of the mass, with the less enhanced portion in the medial peripheral portion (arrows in C); this portion corresponds to the hypocellular collagenous sclerotic area found at pathologic correlation.
E, Low-power photomicrograph shows a well-circumscribed mass adjacent to a minor salivary gland in the buccal wall. The peripheral portion (P) is hypocellular but more collagenous (blue, arrows) compared with the central portion (C); this finding correlates with the difference in signal intensity between the central and peripheral portions on the T2-weighted and contrast-enhanced T1-weighted images (Masson-trichrome, magnification × 40).
F, Photomicrograph shows that the tumor is composed of mostly a hypercellular proliferation of spindle cells arranged haphazardly in a collagenous background. The spindle cells show elongated bland nuclei surrounded by a rim of amphophilic cytoplasm (hematoxylin-eosin, magnification × 200). The inset shows that the tumor cells and capillary endothelial cells have immunohistochemically positive CD-34 results; this finding is consistent with that of a solitary fibrous tumor (avidin-biotin conjugation, magnification × 200).
Excision of the mass was performed by means of an intraoral approach. A 2 × 3 cm, well-circumscribed, unencapsulated, ovoid mass was removed without much difficulty. The mass was whitish, rubbery, and firm. Microscopically, the lesion was composed of spindle-shaped cells with a patternless arrangement in a collagenous matrix. Numerous blood vessels and dilated vascular spaces were present, with an occasional hemangiopericytoma-like pattern (Fig 1E and F). Immunohistochemical studies showed positive staining of the tumor cells for vimentin, but no staining for cytokeratin, S-100 protein, desmin, or actin. In addition, the tumor showed strong CD34 immunoreactivity (Fig 1F), which is a specific marker for solitary fibrous tumor and noted for its absence in other epithelial tumors. Therefore, the diagnosis was a solitary fibrous tumor.
Discussion
The histogenesis of solitary fibrous tumor has been controversial, but findings of a recent immunohistochemical and ultrastructural analysis has strongly suggested an origin of submesothelial mesenchymal fibroblast-like cells, rather than epithelial or mesothelial tissue (4). The ideas of this mesenchymal, rather than mesothelial, origin was further supported by the occurrence of this tumor in extrapleural sites (such as the peritoneum, pericardium, lung, liver, meninges, spinal epidural and/or intradural space, sublingual gland, mediastinum, thyroid, and orbit) and in the upper respiratory tract (including the nasal fossa, nasopharynx, paranasal sinuses, and parapharyngeal space), some of which are devoid of mesothelium (4).
The reported CT findings of solitary fibrous tumors were those of a well-defined soft-tissue mass with heterogeneously or homogeneously strong enhancement (1, 4–6) and occasional calcification or necrosis (4, 5).
On MR images, solitary fibrous tumors were mostly isointense to the muscle on T1-weighted images and hypointense on T2-weighted images, and they showed heterogeneous or homogeneous enhancement (1, 4, 6–8). In this case, the hyperintensity on T2-weighted images was rather unusual compared with the hypointensity usually reported. At pathologic correlation, this portion corresponded well to the hypocellular but more collagenous sclerotic area.
The radiologic differential diagnosis for a neoplasm in the buccal space includes tumors of minor salivary gland origin (benign tumors, such as pleomorphic adenoma, are most common), soft-tissue sarcoma, and lymphadenopathy. Other less common tumors in the buccal space include lipoma, lymphoma, nerve sheath tumor, and hemangioma. Most lesions have attenuation and signal intensity similar to that of muscle on CT and T1-weighted images, respectively; on T2-weighted images, the lesions have signal intensity greater than that of muscle. Additionally, on both enhanced CT and contrast-enhanced T1-weighted images, most lesions enhance moderately or strongly, usually in a homogeneous fashion. Therefore, most buccal space masses have a nonspecific imaging appearance (although lymphadenopathy and some minor salivary gland tumors occasionally have rim enhancement), hemangiomas occasionally have characteristic phleboliths, and lipomas have typical fat attenuation or signal intensity (9, 10). Therefore, to establish a histologic diagnosis, biopsy is mandatory in most cases.
Pathologically, solitary fibrous tumors must be differentiated from other benign soft-tissue tumors, such as neurogenic tumor, fibroblastic tumor, or hemangiopericytoma (1). However, CD34, a transmembrane glycoprotein expressed by hematopoietic progenitor cells, endothelium, and certain populations of mesenchymal stromal cells in the dermis, has been proposed as the most sensitive marker for solitary fibrous tumors (11). When used in conjunction with histopathologic findings and immunohistologic studies, CD34 is useful for discriminating solitary fibrous tumor from other tumors (5).
Most cases of solitary fibrous tumor, especially those at extrapleural sites, are benign and cured with complete surgical excision. Malignant solitary fibrous tumors with an invasive growth pattern, cellular pleomorphism, and abundant mitotic activity frequently recur and may metastasize (12). In malignant solitary fibrous tumors, the resectability seems to be the most important prognostic factor. Some authors (13) have reported good results with radiation therapy or chemotherapy for incompletely resected tumors.
Footnotes
Address reprint requests to Ji Hoon Shin, M.D., Department of Diagnostic Radiology, Ulsan University Hospital, 290–3 Junha-Dong, Dong-Gu, Ulsan 682–714, South Korea.
References
- 1.Iwai S, Nakazawa M, Yoshikawa F, Amekawa S, Sakuda M. Solitary fibrous tumor of the buccal mucosa: report of a case with immunohistochemical studies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;88:461-465 [DOI] [PubMed] [Google Scholar]
- 2.Kurihara K, Mizuseki K, Sonobe J, Yanagihara J. Solitary fibrous tumor of the oral cavity: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:223-226 [DOI] [PubMed] [Google Scholar]
- 3.Perez-Ordonez B, Koutlas IG, Strich E, Gilbert RW, Jordan RC. Solitary fibrous tumor of the oral cavity: an uncommon location for a ubiquitous neoplasm. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999;87:589-593 [DOI] [PubMed] [Google Scholar]
- 4.Kim TA, Brunberg JA, Pearson JP, Ross DA. Solitary fibrous tumor of the paranasal sinuses: CT and MR appearance. AJNR Am J Neuroradiol 1996;17:1767-1772 [PMC free article] [PubMed] [Google Scholar]
- 5.Festa S, Lee HJ, Langer P, Klein KM. Solitary fibrous tumor of the orbit: CT and pathologic correlation. Neuroradiology 1999;41:52-54 [DOI] [PubMed] [Google Scholar]
- 6.Sato J, Asakura K, Yokoyama Y, Satoh M. Solitary fibrous tumor of the parotid gland extending to the parapharyngeal space. Eur Arch Otorhinolaryngol 1998;255:18-21 [DOI] [PubMed] [Google Scholar]
- 7.Nikas DC, De Girolami U, Folkerth RD, Bello L, Zamani AA, Black PM. Parasagittal solitary fibrous tumor of the meninges: case report and review of the literature. Acta Neurochir (Wien) 1999;141:307-313 [DOI] [PubMed] [Google Scholar]
- 8.Vorster SJ, Prayson RA, Lee JH. Solitary fibrous tumor of the thoracic spine: case report and review of the literature. J Neurosurg 2000;92:217-220 [DOI] [PubMed] [Google Scholar]
- 9.Kwong MD, Bert RJ. Neuroradiology case of the day: polymorphous low-grade adenocarcinoma of a minor salivary gland in the right buccal space. AJR Am J Roentgenol 1999;173:805,808-809 [DOI] [PubMed] [Google Scholar]
- 10.Tart RP, Kotzur IM, Mancuso AA, Glantz MS, Mukherji SK. CT and MR imaging of the buccal space and buccal space masses. Radiographics 1995;15:531-550 [DOI] [PubMed] [Google Scholar]
- 11.van de Rijn M, Lombard CM, Rouse RV. Expression of CD34 by solitary fibrous tumors of the pleura, mediastinum, and lung. Am J Surg Pathol 1994;18:814-820 [DOI] [PubMed] [Google Scholar]
- 12.Chan JK. Solitary fibrous tumor—everywhere, and a diagnosis in vogue. Histopathology 1997;31:568-576 [DOI] [PubMed] [Google Scholar]
- 13.Goodlad JR, Fletcher CD. Solitary fibrous tumor arising at unusual sites: analysis of a series. Histopathology 1991;19:515-522 [DOI] [PubMed] [Google Scholar]


