Abstract
Summary: A new angioscopic technique with a CO2 gas medium for prolonged viewing sessions in the carotid artery is described. A stationary column of CO2 gas, angled 17–30° subhorizontally and buoyed against a balloon catheter, can be safely maintained. During 10–20-min sessions in dogs, endothelia, thrombi, stent filaments, coils, and an intimal flap were visualized. This technique eliminates the need for continuous saline infusion, which has prevented the application of angioscopy in the carotid artery.
Angioscopy with a saline flush enables transient endoluminal video imaging during coronary and peripheral revascularization procedures. In comparison, transient CO2 infusion angioscopy has produced images of comparable or superior quality in the peripheral canine circulation (1, 2). CO2 also has served as a nontoxic, nonallergenic, negative angiographic contrast medium. However, results in animal studies regarding the safety of large-volume CO2 injection into the cerebral circulation have varied (3–5).
We intended to develop a new technique to facilitate prolonged angioscopic visualization without saline infusion and to test it in vivo in canine carotid arteries. This could be accomplished, we thought, by establishing a static column of CO2 gas buoyed against an occlusion balloon catheter and with antigravitational arterial positioning. We also intended to demonstrate that CO2 could serve as a safe angioscopic medium in the carotid arteries.
Description of Technique
Our experiments were conducted in two phases. Phase I was a safety and feasibility study for the use of CO2 in pigs and dogs. Phase II was an imaging trial in dogs. All animals were treated according to the ethics guidelines of the University at Buffalo, State University of New York Animal Care and Use Committee. Percutaneous catheterization of the femoral artery was performed by means of a Seldinger puncture. All animals received heparin (150 U/kg). Devices were guided by using digital subtraction angiography (DSA) with an iodinated contrast medium (Toshiba America Medical Systems).
CO2 Column
An occlusion balloon catheter with an inner diameter of 0.096 inches was inflated in the common carotid artery to block proximal blood flow. The animal's neck was positioned downward at varying subhorizontal angles by elevating the proximal aspect of the neck with cushions. Then, 0.5 mL of CO2, followed by a saline column, was injected through the balloon lumen with a syringe until a gas column became visible on fluoroscopic images (Fig 1). The gas was buoyed against the balloon because of the subhorizontal position of the carotid artery, and the pressure of the saline in the catheter stabilized the distal gas-blood interface. During phase II, the imaging trial, a flexible angioscope (diameter, 0.96 mm; Elekta) connected to a video monitoring system (Endoview; Imagyn Medical Technologies, Irvine, CA) with a high-intensity light source was advanced through the lumen of the balloon catheter. When the tip of the angioscope was beyond the balloon, the CO2 was introduced, establishing a gas column with the tip inside it. All gas remaining in the column at the end of each experiment was withdrawn through the lumen of the balloon catheter into a syringe and discarded. The balloon was then deflated and removed.
fig 1.
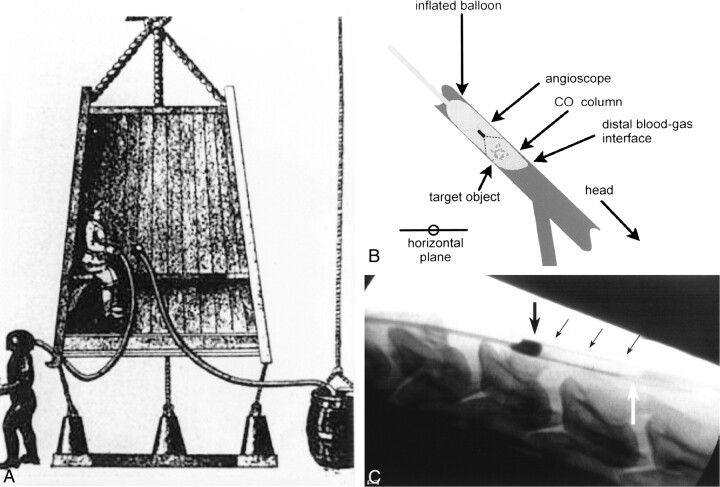
Principles of the technique.
A, After predicting the appearance of a periodic comet, Sir Edmund Halley, in 1716, proposed that a diving bell could provide a pressurized compartment for deep-sea exploration. Air could not escape because the bell was closed at the top and sides. Water could not enter at the open bottom because buoyant air was trapped inside the bell, forming a stable chamber.
B, Our device traps a small column of CO2 in an artery by blocking proximal blood flow with a balloon and by positioning the target artery subhorizontally. The injected gas evacuates blood and buoys itself against the balloon.
C, The column of CO2, a negative contrast medium, is visible at fluoroscopy (arrows).
Phase I
The small-diameter vascular network of the porcine rete mirabile, originating from a distal branch of the common carotid artery, provided a site for convenient and safe simulation of human intracerebral arterioles to visualize gas transit radiographically. Use of the pig rete in this phase helped to prevent acute stroke as a complication of gas embolism of brain arteries. In two pigs, we injected 2 mL of CO2 into the rete mirabile. Two control pigs received the same amount of room air. Vital parameters—blood pressure, mean arterial pressure, and pulse and respiratory rates—were continuously monitored. Blood flow rates in the rete were monitored by measuring radiographic contrast material transit times at the time of CO2 injection and 1, 3, and 5 min thereafter.
In one pig and one dog, we tested the angle of the carotid artery that was required to keep the gas column buoyant against the proximally inflated balloon catheter by moving the animal's head in declinations from 30° to 5°. After realizing that retrograde flow from small vessels originating in the pig common carotid artery (inferior and superior thyroid arteries and a few muscular branches) disrupted the gas column, we decided to shift our testing to a canine model in which these vessels are usually absent. In addition, most humans lack side branches arising from the proximal internal carotid artery; these factors made the dog model a better paradigm for clinical simulation than did the pig model.
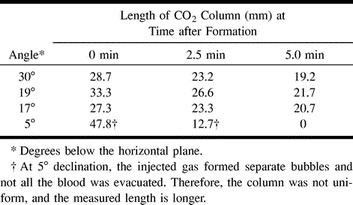
The steel edge of the angiography table served as our horizontal reference. Because our angiography table was fixed in the horizontal position, we placed sandbags beneath the shoulders of each animal to achieve various angulations of the carotid artery. The angles between the target carotid artery and the horizontal reference were then measured on angiographic images. We also measured the initial length of the gas column and its length 2.5 and 5.0 min after CO2 injection. From this data, the rate at which the gas column shrank was calculated for each angle. While the gas column was in place, we watched it closely and noted any visible distal migration of gas or bubbling around the column.
Phase II
A CO2 column was established in the carotid arteries of three dogs with a 20° subhorizontal angle and 0.5 mL of CO2. In each animal, gas columns were established for 10–20 min at a time. The angioscope was moved within each gas column in both anterograde and retrograde directions, and images were simultaneously displayed on a color monitor and recorded to a VHS videotape.
In the first dog, a healthy carotid intima was surveyed with the angioscope. Then a helical platinum coil was deployed in the lumen to induce a thrombotic occlusion. Fifteen minutes later, the fresh thrombus was visualized with the angioscope. In the second dog, a self-expanding nitinol stent (Integra; Boston Scientific Corporation/SCIMED, Natick, Mass) was deployed prior to the imaging session, and the stent was then contained within the CO2 column. In the third dog, a side-wall venous pouch aneurysm was surgically grafted to the common carotid wall and later packed with Guglielmi detachable coils (GDCs). One month after coil packing, the neck of the aneurysm, with coils protruding, was visualized with angioscopy. Intravascular sonography also was performed.
Results
Phase I
After CO2 injection, no substantial changes in vital parameters were observed. Within 1 min of the injection, the flow rate of contrast material through the rete was slowed but returned to baseline within 2–5 min. No angiographic evidence of CO2 remained. In both control animals, seizures developed within 1 min of the injection of room air, and flow through the rete was stagnant or suboptimal for at least 10 min. No vital parameters changed in the control animals.
When the common carotid artery was angled 17–30° subhorizontally, a stationary column of CO2 gas was maintained for 20 min in a dog and a pig. A small amount of gas slowly dissolved at the distal gas-blood interface at angles of 17° or greater (Table). However, at 5°, separate CO2 bubbles formed, and some gas escaped to the distal circulation. At angles of 17° or greater, the rate of column shortening was 3–5 mm/min. At 5°, this rate increased to 20–30 mm/min. No vital parameter changes or seizures occurred. The dog, having recovered from anesthesia, had no neurologic deficits.
Length of CO2 column as a factor of time and arterial declination, as measured in a pig
Phase II
A stable CO2 column, which gradually shrank, was established in the three dogs for 10–20 min. Images were obtained as the angioscope was either advanced or withdrawn, but steering the scope was difficult. In the first dog, the endothelium and the induced thrombus were displayed clearly by using the angioscope (Fig 2). In the second dog, each filament of the stent was visible at angioscopy (Fig 3), while intravascular sonograms showed only the reflected outlines of the stent, without details. In the third dog, angioscopic images showed protruding coils with a transparent endothelial covering (Fig 4). It also revealed an intimal flap (Fig 5), which DSA and intravascular sonography did not.
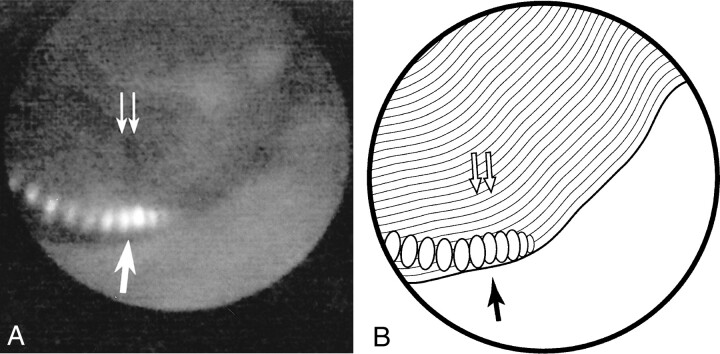
fig 2.
Experimentally induced arterial occlusion.
A, The occlusion, with a helical platinum coil (arrow), is clearly depicted as a thrombus (double arrows) at angioscopy.
B, Line diagram of image in A.
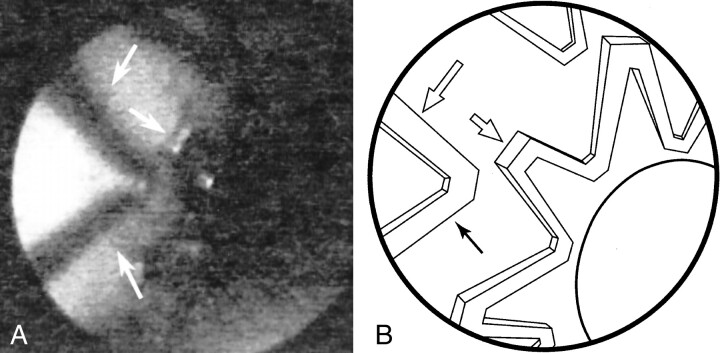
fig 3.
Stent deployment.
A, A self-expanding nitinol stent (Integra; Boston Scientific/SCIMED) was deployed in the carotid artery. Angioscopic image of the CO2 column shows each stent filament in detail (arrows) and confirms the stent was successfully deployed into the arterial wall.
B, Line diagram of image in A.
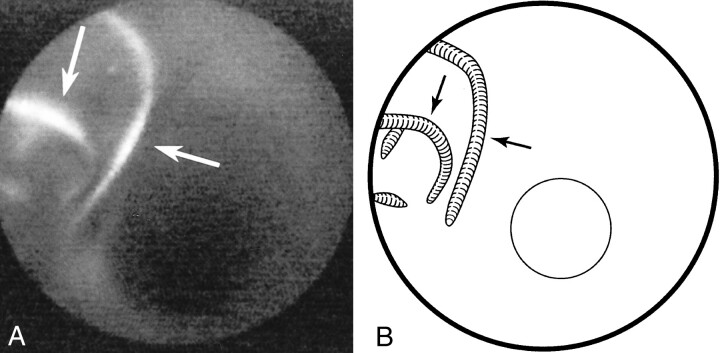
fig 4.
GDC placement.
A, Several GDCs were used to pack an experimental venous pouch aneurysm grafted to the side of the carotid arterial wall. One month later, an angioscopic image shows the protruding coils (arrows), which have a transparent endothelial covering.
B, Line diagram of image in A.
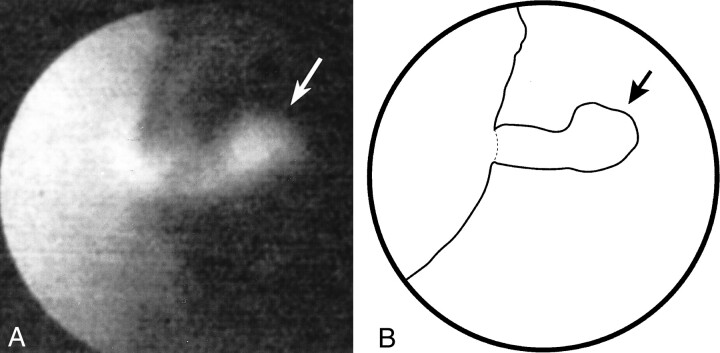
fig 5.
Depiction of the intimal flap.
A, Angioscopic image shows the intimal flap (arrow) that was not visible at DSA or intravascular sonography.
B, Line diagram of image in A.
Discussion
CO2 as an Angioscopic Medium
Maintaining a blood-free field of observation with the saline infusion method has been the major obstacle of clinical angioscopy (2, 6, 7). Large volumes and sophisticated injection systems often are used, because blood quickly mixes with saline to distort the image. Saline loads are especially hazardous in patients with heart failure or renal insufficiency, and they may cause pulmonary edema (1, 2, 8). Also, the cerebral circulation is particularly susceptible to ischemia after direct large-volume saline infusion.
A CO2 flush has been used in dogs to obtain high-resolution angioscopic images (1, 2). CO2 evacuates blood without the mixing caused by saline (1, 2, 9). Silverman et al (1) infused CO2 at a rate of 2 mL/s into the femoral artery of dogs against anterograde flow. In 20 of 25 animals, a clear visual field for angioscopy was established within 8 s and was maintained for as long as 25 s during gas infusion and 10 s or longer after the infusion was stopped. These results demonstrate the ability of CO2 to evacuate flowing blood to create a visual field. However, this evacuation was accomplished with a relatively large volume of gas (80 mL), which was not removed from the circulation.
CO2 has been used clinically in thousands of patients as a negative contrast medium for angiography without notable complications or discomfort (9, 10). It is colorless, odorless, and noncombustible, and it has a very low viscosity (1, 9). CO2 is deliverable through tiny microcatheters and angioscopic flush channels (1, 2). It is 20 times more soluble than oxygen in blood at 38.0°C and rapidly combines with blood buffers; it is then expired (1, 9–11). Hawkins et al (12) demonstrated that infused CO2 clears from the renal arteries of dogs in less than 6 s and does not cause endothelial changes.
Shifrin et al (3) used 120–300 mL of CO2 in dogs for carotid angiography and observed no neurologic, electroencephalographic, or carotid filling changes. Coffey et al (4), however, reported morbidity and mortality after injecting 0.25–1.5 mL of CO2 into the internal carotid arteries of rats. After injecting 280–850 mL of CO2 into the internal carotid arteries of mini-pigs, Linstedt et al (5) observed cerebral edema, electroencephalographic changes, and (in two of six animals) nerve cell damage. Despite these inconsistencies, we believe the findings in the latter two studies do not impugn ours. We injected 0.5 mL of CO2, which was continuously buoyed against a balloon and was removed at the end of each viewing session. A small amount dissolved (either through the endothelium, at the distal gas-blood interface, or both), but only balloon rupture or position change could have caused the escape of gaseous CO2. Even so, it is unclear whether this is harmful. Although we saw no evidence of CO2 toxicity, we believe that further study of the safety of low-dose CO2 in the carotid artery is necessary but that its safety in the peripheral circulation is well established.
Advantages of CO2 Column Angioscopy
Direct visualization of the endoluminal surface at angioscopy is an established tool in vascular procedures. In the coronary arteries, angioscopy has been used in all phases of lesion stent placement (13). It has been most useful for distinguishing thrombotic from nonthrombotic occlusions. Angioscopy may be useful in selecting additional stents or thrombolytic therapy and in predicting restenosis. Some have said that it is superior to angiography and intravascular sonography for the depiction of thrombi, dissection, and friable plaques in venous grafts. Diethrich et al (7) described the use of angioscopy in the lower extremities of 23 patients to select their revascularization technique. Angioscopy led to the lysis of three subacute arterial thromboses. In four cases, angioscopy revealed incomplete recanalization despite satisfactory angiographic images. Angioscopy also facilitated passage of probes through tight stenoses.
Carotid revascularization with angioplasty and stent procedures is emerging as a safe and effective, but much less invasive, alternative to endarterectomy in select patients (14). We believe that CO2 column angioscopy could become a powerful tool for carotid and peripheral revascularization because it can be used to show diseased segments and guide wires, stents, and other endovascular devices. The saline method provides less than 5 s of viewing, yet we could easily maintain a CO2 column for at least 20 min in this study. The proximal and distal circulation blocks, accomplished with the balloon and gas column, respectively, provided a motion-free environment for the angioscope that was not achievable with the saline infusion method. Blood supply to the occluded territory of the brain is maintained via collateral flow from the circle of Willis. Thrombosis was avoided by administering heparin to each animal.
Compared with saline, CO2 is a safer and more effective medium for laser ablation of atherosclerotic plaques (1, 9). Column angioscopy could be used to guide laser angioplasty, which has failed largely because of an inability to direct the beam (15). Furthermore, we have previously suggested that accurate identification of carotid plaque ulceration might be an important step in stroke prevention (16). Column angioscopy also would be an excellent tool for this objective. In this study, angioscopy revealed the detailed structure of stents, intimal and thrombotic coverage of platinum coils, and an intimal flap not seen at DSA or intravascular sonography.
Limitations of the Study
The angioscopic catheter that we used provided relatively weak illumination. Consequently, the periphery of the images appeared dark, which made orientation to still shots more difficult than the on-line images. The quality of still video images (source of our prints) is related to the low-light environment and the video noise of the camera. Viewing the VHS videotape as a movie reduces video noise and provides much better image quality.
We used our technique at arterial locations without side branches. The CO2 column might not be able to withstand the retrograde pressure of side branches receiving collateral flow, which hinders the saline infusion method (2, 8). Insufficient angulation may cause the CO2 to form separate bubbles. Also, a small amount of CO2 dissolves, gradually shortening the column. Although we were able to move the angioscope forward and backward, steering was difficult. Compared with angiography and intravascular sonography, our system uses an occlusion balloon and cannot depict the media or adventitia (intravascular sonography can). Also, patients without sufficient collateral flow (because of an incomplete circle of Willis) may not tolerate lengthy carotid occlusion.
Conclusion
We used a column of CO2 gas as a contrast medium for angioscopy in canine carotid arteries. We viewed, in color, the endothelia, stents, GDCs, thrombi, and intimal flap during 10–20-min sessions.
Acknowledgments
We thank Paul Dressel for his preparation of the figures; Dean Marky and Mike Rejewski for their help with the CO2 setup; Beth Golebiewski and Imagyn Medical Technologies for providing a video monitoring system; Doug Garrabrant and Elekta for providing a video endoscope coupler; and Doug Killian, Liz Stejskal, and Boston Scientific Corporation/SCIMED for providing stents for this study.
Footnotes
This work was presented in part at the 36th Annual Meeting of the American Society of Neuroradiology, May 1998, Philadelphia, PA.
Address reprint requests to L. Nelson Hopkins, MD, Department of Neurosurgery, Millard Fillmore Hospital, 3 Gates Circle, Buffalo, NY 14209.
References
- 1.Silverman SH, Mladinich CJ, Hawkins IF Jr, Abela GS, Seeger JM. The use of carbon dioxide to displace flowing blood during angioscopy. J Vasc Surg 1989;10:313-317 [PubMed] [Google Scholar]
- 2.Mladinich CRJ, Akins EW, Wengarten KE, Hawkins IF Jr. Carbon dioxide as an angioscopic medium: comparison to various methods of saline delivery. Invest Radiol 1991;26:874-878 [DOI] [PubMed] [Google Scholar]
- 3.Shifrin EG, Plich MB, Verstandig AG, Gomori M. Cerebral angiography with gaseous carbon dioxide. J Cardiovasc Surg 1989;31:603-606 [PubMed] [Google Scholar]
- 4.Coffey R, Quisling RG, Mickle JP, Hawkins IF Jr, Ballinger WB. The cerebrovascular effects of intraarterial CO2 in quantities required for diagnostic imaging. Radiology 1984;151:405-410 [DOI] [PubMed] [Google Scholar]
- 5.Linstedt U, Link J, Grabener M, Kloess W. Effects of selective angiography of the carotid artery with carbon dioxide on electroencephalogram somatosensory evoked potentials and histopathologic findings: a pilot study in pigs. Invest Radiol 1997;32:507-510 [DOI] [PubMed] [Google Scholar]
- 6.Haas R. Implantation and imaging of coronary stents. Radiol Technol 1996;67:233-244 [PubMed] [Google Scholar]
- 7.Diethrich EB, Yoffe B, Kiessling JJ, et al. Angioscopy in endovascular surgery: recent technical advances to enhance intervention selection and failure analysis. Angiology 1992;43:1-10 [DOI] [PubMed] [Google Scholar]
- 8.White JV, Eid I. Diagnostic and interventional angioscopy. Surg Clin North Am 1998;78:539-559 [DOI] [PubMed] [Google Scholar]
- 9.Yang X, Manninen H, Soimakallio S. Carbon dioxide in vascular imaging and intervention. Acta Radiol 1995;36:330-337 [PubMed] [Google Scholar]
- 10.Kerns SR, Hawkins IF Jr. Carbon dioxide digital subtraction angiography: expanding applications and technical evolution. AJR Am J Roentgenol 1995;164:735-741 [DOI] [PubMed] [Google Scholar]
- 11.Kuo PC, Petersen J, Semba C, Alfrey EJ, Dafoe DC. CO2 angiography: a technique for vascular imaging in renal allograft dysfunction. Transplantation 1996;61:652-654 [DOI] [PubMed] [Google Scholar]
- 12.Hawkins IF Jr, Miadinich CR, Storm B, et al. Short-term effects of selective renal arterial carbon dioxide administration on the dog kidney. J Vasc Interv Radiol 1994;5:149-154 [DOI] [PubMed] [Google Scholar]
- 13.Annex BH. Coronary angioscopy: clinical applications. Cardiol Clin 1997;15:131-137 [DOI] [PubMed] [Google Scholar]
- 14.Yadav JS, Roubin GS, Iyer S, et al. Elective stenting of the extracranial carotid arteries. Circulation 1997;95:376-381 [DOI] [PubMed] [Google Scholar]
- 15.Ferris EJ, Lebor K, ben-Avi DD, et al. Percutaneous angioscopy: work in progress. Radiology 1985;157:319-322 [DOI] [PubMed] [Google Scholar]
- 16.Miskolczi L, Guterman LR, Flaherty JD, Hopkins LN. Depiction of carotid plaque ulceration and other plaque-related disorders by intravascular sonography: a flow chamber study. AJNR Am J Neuroradiol 1996;17:1881-1890 [PMC free article] [PubMed] [Google Scholar]