Abstract
BACKGROUND AND PURPOSE: Aneurysmal rupture during endovascular treatment is one of the most feared complications of endovascular aneurysm therapy. The purpose of this study was to determine the frequency, causes, management, and outcome of aneurysmal rupture that occurred during treatment with Guglielmi detachable coils (GDCs) in an unselected series of patients with ruptured cerebral aneurysms.
METHODS: Between July 1997 and December 2000, we treated 164 acutely ruptured cerebral aneurysms with GDCs. All charts were reviewed, and patients with aneurysmal rupture occurring during embolization were identified.
RESULTS: Five patients had an intraprocedural aneurysmal rupture. In one patient, rupture was due to guidewire perforation of the wall. In two patients, the microcatheter itself perforated the aneurysm. In another two patients, rupture occurred during placement of the first coil. Endovascular packing was continued in all patients. One patient died as a result of the aneurysmal rupture. No negative long-term effects were observed in the remaining four patients. In summary, we observed intraprocedural aneurysmal rupture in 3% of our patients, with a mortality rate of 20% and no long-term morbidity.
CONCLUSION: Aneurysmal rupture during endovascular treatment with GDCs is a rare event; clinical severity may be variable. Embolization of the aneurysm can be continued in most cases, and most patients with treatment-related subarachnoid hemorrhage survive without serious sequelae.
Endovascular treatment of intracranial aneurysms is rapidly undergoing major developments. Initial techniques of selective balloon occlusion were progressively replaced by controlled packing of the aneurysmal sac with detachable coils (1–3). Technical improvements in catheters and the advent of Guglielmi detachable coils (GDCs) allow routine entry into and direct obliteration of many aneurysms, with acceptable morbidity and mortality rates and an efficacy comparable with that of surgical treatment (3–6).
Despite these advances, aneurysm rupture during endovascular treatment has continued to be one of the most feared complications of endovascular aneurysm therapy. Although any interventional neuroradiologist who treats acutely ruptured aneurysms can face this complication, few data regarding frequency, causes, management, and outcome of such rupture during endovascular treatment are available (7–11). Our purpose was to determine the frequency, causes, management, and outcome of aneurysmal rupture during treatment with GDCs in an unselected series of patients with ruptured cerebral aneurysms.
Methods
Between July 1997 and December 2000, 164 ruptured cerebral aneurysms were treated with GDCs at our institution. Treatment was performed as soon as possible after admission, with all patients under general anesthesia. All patients routinely underwent control CT within 3 d after treatment.
For this study, all charts of our aneurysm database were reviewed, and five patients who had aneurysm rupture during embolization were identified. Diagnosis of rupture was made on the basis of angiographic visualization of contrast material extravasation in two cases, visualization of the first coil partially outside the aneurym in one case, and fluoroscopic visualization of the tip of the microcatheter outside the aneurysm in two cases. The Table presents the clinical data, causes, and outcome (Glasgow Outcome Scale scores) of the five patients who had aneurysm rupture during embolization.
TABLE 1:
Clinical data, causes, and outcomes in five patients with aneurysm rupture during embolization
Results
Five patients had a procedure-related aneurysmal rupture during endovascular treatment. In one patient, the rupture was caused by guidewire perforation of the aneurysmal wall. In two patients, the microcatheter itself perforated the aneurysm. In another two patients, the rupture occurred during placement of the first coil. Endovascular packing was continued in all patients. One patient died as a result of massive bleeding after aneurysmal rupture; in the remaining four patients, no negative long-term effects were observed. Overall, we observed an intraprocedural aneurysmal rupture rate of 3%, with a mortality rate of 20% and no long-term morbidity.
Case 1
A 24-year-old man presented with acute headache and mild meningism. CT revealed no subarachnoid hemorrhage (SAH). However, SAH was confirmed with lumbar puncture and was clinically classified as Hunt and Hess grade 1. Initial diagnostic angiograms (at day 1) showed no aneurysm. Repeat angiography performed 21 d after SAH revealed a wide-necked aneurysm of the posterior communicating artery (Fig 1). The patient was referred for endovascular therapy. The aneurysm was selectively catheterized by using a FasTracker-18 microcatheter and a Transend EX-14 guidewire (Boston Scientific/Target, Fremont, CA). The tip of the microcatheter was shaped by using steam to enable stable positioning within the aneurysm. During delivery of the first coil (GDC-18, 3 × 4; Boston Scientific/Target), rupture of the aneurysm occurred, with a tear at the aneurysm base and massive extravasation of contrast material (Fig 1). Within a few seconds, arterial blood pressure dramatically increased to 22/110 mm Hg. We decided to detach the first coil, but SAH was not controllable. No additional coils were placed, and the procedure was stopped. The patient was immediately transferred to the operating room for surgical clip placement. Craniectomy revealed massive hemispheric swelling and SAH. Surgical clip placement was successful, but the patient died 1 d later as a result of massive brain edema (Fig 1).
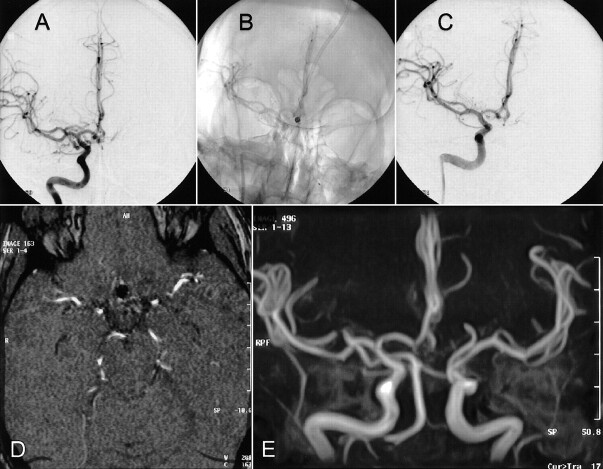
fig 1.
Case 1.
A, Lateral projection arteriogram of the left internal carotid artery reveals a posterior communicating artery aneurysm.
B, Lateral projection arteriogram of the left internal carotid artery, obtained immediately after aneurysm rupture that occurred after placement of the first coil, shows the rupture and massive extravasation of contrast material.
C, Selective angiogram obtained via the microcatheter.
D, Axial view CT scan obtained after clip placement in the aneurysm shows massive brain edema and contrast agent in the subarachnoid space.
Case 2
A 26-year-old man presented with SAH (Hunt and Hess grade 1) that had occurred 5 d previously. Angiography revealed an aneurysm of the basilar tip and no vasospasms (Fig 2). A FasTracker-18 microcatheter and a Terumo-GT microguidewire (Terumo, Natick, MA) with a 45° angled flexible tip were used for selective catheterization of the aneurysm. While advancing the microcatheter over the wire in the aneurysm, the microguidewire advanced further, and it perforated the aneurysm dome (Fig 2). The microguidewire was pulled back within the aneurysm, the microcatheter was advanced further in the aneurysm, and embolization of the aneurysm was subsequently successfully performed by using three GDCs (GDC-18; 6/20, 5/20, 2/4). Perforation was confirmed with slight extravasation of contrast material and/or blood depicted on the angiogram and subsequently on CT scans (Fig 2D). Because we observed no extravasation of contrast material after placement of the third coil, anticoagulation therapy (a bolus of 5000 U of IV heparin) was initiated and maintained for 24 h. The clinical condition remained unchanged, with no new neurologic symptoms. Two days after endovascular treatment, ventriculostomy was performed because of slight hydrocephalus. Subsequently, aseptic meningitis developed, and the patient had a good recovery. The final outcome was good (Glasgow Outcome Scale score of 1).
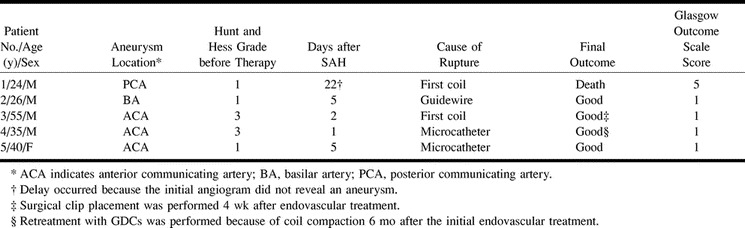
fig 2.
Case 2.
A, Frontal projection arteriogram of the left vertebral artery shows a basilar tip aneurysm.
B, Frontal projection arteriogram of the right vertebral artery, obtained after placement of two GDCs, reveals slight extravasation of contrast material (magnification).
C, Frontal projection arteriogram of the left vertebral artery shows complete embolization of the basilar artery tip aneurysm.
D, Axial view CT scan depicts slight extravasation of contrast agent and blood.
Case 3
A 55-year-old man presented with acute SAH (Hunt and Hess grade 3), and CT revealed additional intraventricular hemorrhage and hydrocephalus. Subsequent angiography revealed a 2-mm aneurysm of the anterior communicating artery (Fig 3). Endovascular therapy was administered immediately after diagnostic angiography. The aneurysm was selectively catheterized by using an Excel-14 microcatheter (Boston Scientific/Target) and a Transend EX-14 guidewire. A coil (GDC-10, soft 2 × 2) was selected for embolization. During coil placement, the coil perforated the aneurysm wall and partially protruded into the subarachnoid space (Fig 3). The other part of the coil filled the small aneurysm sac. The coil was left in place and not pulled back within the aneurysm. On the basis of a subsequent control angiogram that showed satisfactory subtotal aneurysm occlusion and only a small aneurysm remnant (Fig 3), we decided to detach the coil. No additional coils were detached. Because of the subtotal occlusion and the subsequently scheduled ventriculostomy, anticoagulation therapy was not initiated. CT performed immediately after the procedure revealed extravasation of contrast material into the subarachnoid space (Fig 3). Ventricular size was unchanged compared with that depicted on the initial CT scan, and a ventriculostomy drain was placed. The clinical condition remained unchanged, with no new neurologic symptoms. Four weeks after endovascular treatment, control angiography was performed; angiograms revealed an aneurysm remnant. The patient was referred for surgical clip placement in the residual aneurysm. Intraoperatively, displacement of the coil out of the aneurysm within the subarachnoid space was clearly visible (Fig 3). The final outcome of the patient was good (Glasgow Outcome Scale score of 1).
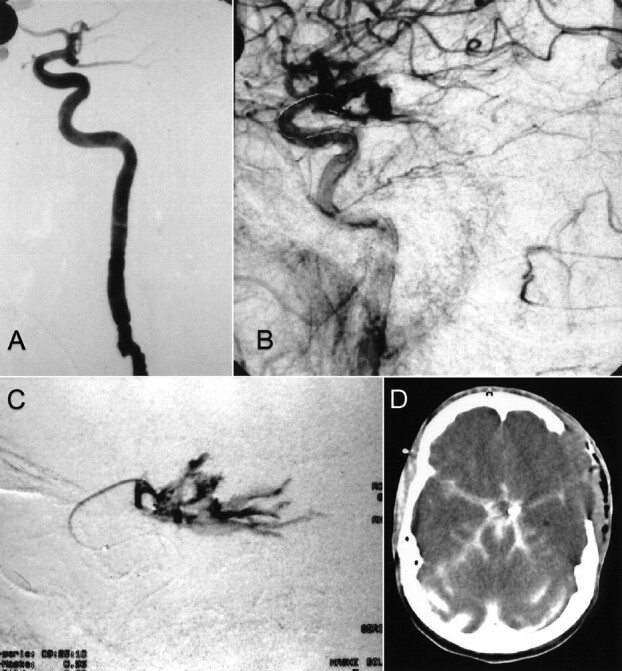
fig 3.
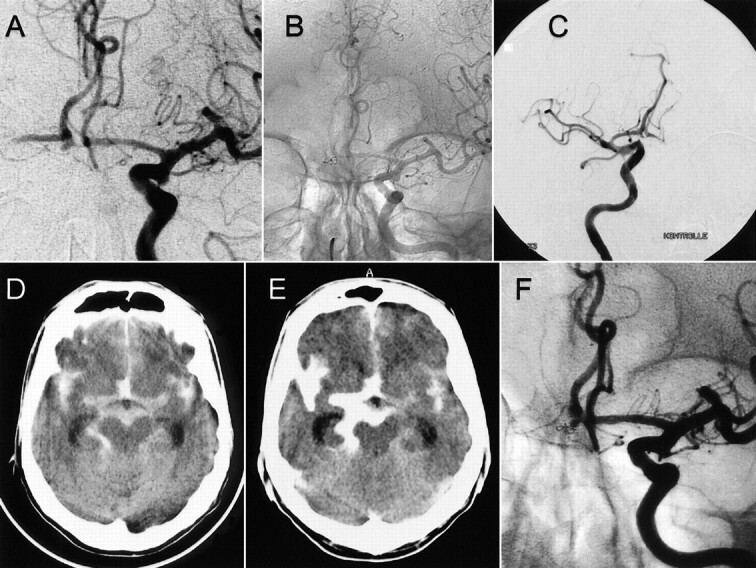
Case 3.
A, Frontal projection arteriogram of the right internal carotid artery shows a small, caudally oriented anterior communicating artery aneurysm.
B and C, Arteriograms of the right internal carotid artery, obtained after placement of one GDC, show that the coil partially protrudes into the subarachnoid space.
D, Axial CT scan, obtained before endovascular treatment, shows SAH and extravasation of contrast material after aneurysm perforation.
E, Axial CT scan, obtained after endovascular treatment, shows SAH and extravasation of contrast material after aneurysmal perforation.
F, Control angiogram, obtained 4 wk after endovascular treatment, reveals the aneurysm remnant.
Case 4
A 35-year-old man presented with SAH, clinically classified as Hunt and Hess grade 3, and was found to have an aneurysm of the anterior communicating artery. Endovascular therapy was performed immediately after diagnostic angiography on day 1 after SAH. With use of an Excel-14 microcatheter and a Transend EX-14 guidewire (Boston Scientific/Target), the aneurysm was selectively catheterized. During withdrawal of the microguidewire, the microcatheter further advanced in the aneurysm, and the tip of the microcatheter perforated the aneurysm dome. Perforation was ascertained by means of fluoroscopic visualization of the tip of the microcatheter outside the aneurysm. We decided to introduce the first coil (GDC-10, 5 × 15) in the microcatheter already advanced to the tip. We then pulled back the microcatheter within the aneurysm, and five coils (GDC-10; 5 × 15, 4 × 10, 4 × 10, 4 × 10, and 4 × 8) were subsequently detached without any problems. Arteriographic studies after placement of each coil performed to document appropriate coil positioning did not reveal any extravasation of contrast agent. Anticoagulation therapy was initiated after placement of the third coil and maintained for 24 h. The final angiogram showed complete occlusion of the aneurysm. CT performed after the procedure revealed no extravasation of contrast material and no new SAH. The clinical condition remained unchanged, and the final outcome was good (Glasgow Outcome Scale score of 1). The patient returned 6 mo later for follow-up. Angiography and MR angiography showed substantial coil compaction, with partial recanalization of the aneurysm. Endovascular therapy was again administered, and the recanalized aneurysm was completely occluded.
Case 5
A 40-year-old woman presented with SAH, clinically classified as Hunt and Hess grade 1, 5 d before admission. Angiography revealed a 4-mm caudally oriented aneurysm of the anterior communicating artery (Fig 4). For selective catheterization, an Excel-14 microcatheter with its tip steamed and a Transend EX-14 guidewire were used. During relief of forward pressure of the microguidewire and simultaneous advancement of the microcatheter into the caudally oriented aneurysm, the microcatheter suddenly was moved forward and perforated the aneurysm dome. We introduced the first coil (GDC-10, 3 × 6) into the microcatheter and advanced it to the tip. The microcatheter was then pulled back into the aneurysm, and embolization of the aneurysm was subsequently performed by using three coils (GDC-10; 3 × 6, 2 × 6, and 2 × 6). Anticoagulation therapy was initiated after placement of the second coil and was maintained for 24 h. Arteriographic studies after placement of three coils showed complete occlusion (Fig 4). The clinical condition remained unchanged, with no new neurologic symptoms (Glasgow Outcome Scale score of 1).
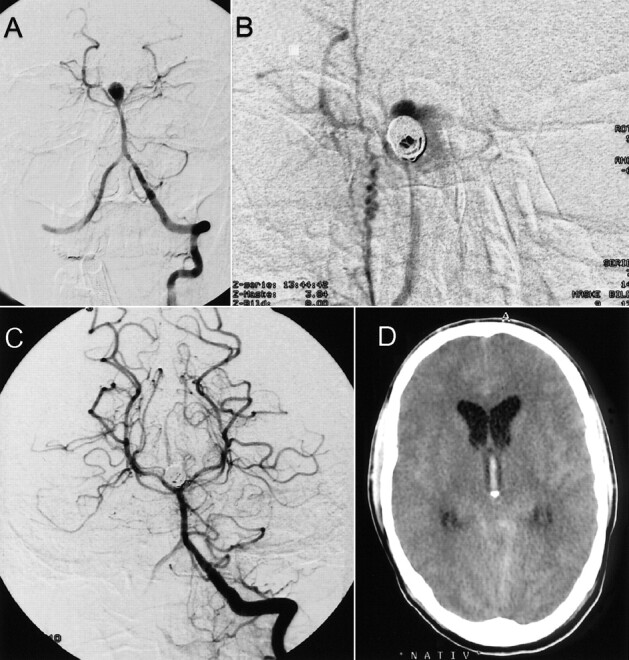
fig 4.
Case 5.
A–C, Frontal projection arteriograms of the right internal carotid artery show an anterior communicating artery aneurysm before and after placement of three GDCs, with complete occlusion of the aneurysm.
D and E, Time-of-flight MR angiograms, obtained 2 d after endovascular treatment, confirm complete occlusion and do not reveal any thrombosed portions of the aneurysm.
Discussion
Implementation of GDCs has substantially changed the management of cerebral aneurysms, making treatment less invasive. However, aneurysm rupture during endovascular treatment can occur and can represent a devastating complication. Many possible mechanisms of aneurysm rupture during treatment exist: Rupture can occur coincidentally during diagnosic angiography or endovascular treatment; generally, it is associated with a poor prognosis (12–14). Increased blood pressure during injection of contrast material can also contribute to rerupture of an acutely ruptured aneurysm (15, 16). Also, during endovascular treatment, rupture of the aneurysm might be due to perforation with the guidewire or microcatheter, or it might occur during coil placement.
Intraoperative aneurysm rupture is one of the most feared complications for the neurosurgeon performing aneurysm surgery. However, intraoperative rupture of aneurysms includes various situations, which range from a minor hemorrhage during clip application to a major rupture during craniotomy (17, 18). Among them, the premature rupture before securing the aneurysm neck generally is the most catastrophic intraoperative complication. In a series of 398 consecutive direct surgical interventions for ruptured cerebral aneurysms, Houkin et al (19) reported 24 cases (6.0%) with a premature intraoperative rupture of the aneurysm. The purpose of our study was to report the frequency, causes, management, and outcome of aneurysmal rupture during GDC treatment of acutely ruptured cerebral aneurysms.
Five of our 164 patients with acutely ruptured aneurysm had procedure-related aneurysm rupture during endovascular treatment. In one case, the rupture was caused by the guidewire. In two cases, it was caused by the microcatheter itself, resulting in a small perforation at the aneurysm dome. As McDougall et al (9) reported, the incidence of uncontrolled advancement of the microcatheter can be decreased by ensuring that no forward pressure is applied to the guidewire and by removing the guidewire slowly under direct fluoroscopic control. Nevertheless, aneurysml rupture due to perforation with the microcatheter might occur, as we observed in two of our cases. Perforations made with the microguidewire (0.014 in) result in small puncture wounds that could close before excessive hemorrhage occurs. In our earlier cases, we sometimes used the Terumo-GT microguidewire. The relative stiffness and sharpness of the tip of this microguidewire might be a contributing factor for aneurysm rupture in our case 2. Therefore, we stopped using this microguidewire for catheterization in endovascular aneurysm treatment.
Perforation caused by the microcatheter that is larger than the guidewire is expected to produce a larger perforation, with more tearing of the aneurysm wall. McDougall et al (9) reported procedural ruptures due to aneurysmal perforation with the microcatheter in two of their four cases, with subsequent CT revealing new SAH, leading to death in one patient and no neurologic deterioration in the second.
As in our cases, consequences of guidewire and microcatheter perforation may be minimal, and in such cases, management involves continuation of embolization. Depending on the clinical severity, coil detachment and subsequent placement of additional coils might be the management of choice if rupture occurs during coil placement. In our two cases with microcatheter perforation, we introduced the first GDC to the tip of the microcatheter before pulling back the microcatheter into the aneurysm to place the coil. Because we visualized microcatheter perforation in our cases 4 and 5 with only fluoroscopy and because we did not observe any extravasation of contrast material from the aneurysm during angiography or subsequent CT, one might argue that the tip of the microcatheter was still located within a thrombosed part of the aneurysm. At our institution, baseline MR angiography during hospitalization is routinely performed for further follow-up in all patients treated endovascularly for cerebral aneurysms. In both patients, MR angiography a few days after endovascular treatment confirmed complete embolization and did not reveal any thrombosed portions of the aneurysm.
An important alternative salvage maneuver in the management of microcatheter perforation during endovascular therapy might be the technique recently described by Willinsky and terBrugge (20) in a case report of a ruptured paraophthalmic aneurysm. With the use of a second microcatheter, the aneurysm is packed with a GDC while the first microcatheter is temporarily left in place. At the end of the coiling procedure, both microcatheters can then be removed. This technique may prevent the hemorrhage that can occur when one attempts to place a coil across the catheter perforation. The authors observed no adverse effects from the procedure, and the outcome was good.
Conversely, consequences of rupture may be devastating, as they were in one of our two cases in which rupture occurred during placement of the first coil. In that case, we observed a tear at the aneurysm base and immediate massive extravasation of contrast material. On the basis of our experience, we think that small aneurysms at the origin of the posterior communicating artery have an increased risk of rupture. Further data based on retrospective analyses of cases may allow better assessment of risk-related anatomy and location and of the results of applied therapeutic management.
In two of our cases, retreatment was necessary during follow-up. In case 3, control angiography performed 4 wk after endovascular treatment revealed an aneurysm remnant, which was treated with surgical clip placement. Endovascular therapy was performed 6 mo after initial treatment in case 4 because of substantial coil compaction. This high frequency of retreatment may be an indicator for our hesitation to pack the previously perforated aneurysm as densely as usual. In these critical cases, we therefore recommend short-term control angiography to prevent missing any substantial aneurysm remnants.
Because the majority of procedure-related complications are of a thromboembolic nature, intraoperative anticoagulation therapy is of great importance (21). In the absence of contraindications, we use intraoperative IV heparin therapy for all patients undergoing endovascular aneurysm treatment. However, in cases of acutely ruptured aneurysms, we usually do not administer heparin before placement of at least the first coil. We then administer a bolus of 3000–5000 U of heparin and then continue infusion to keep the activated clotting time at a level two to three times above normal during treatment. Heparin administration is continued for as long as 24 h; we allow approximately twice the normal value. In cases of complications, heparin administration sometimes is prolonged and intensified. If rupture during endovascular treatment occurs and the aneurysm is not densely packed, heparin therapy may not be initiated or reversed when it already has started. The lack of coils within the aneurysm to promote thrombosis and the relatively long time required to place coils in the aneurysm to try to stop the hemorrhage could result in a poor outcome, as would full heparin administration at the time of hemorrhage. One might question why we administered heparin in our case 2. Because we did not observe any extravasation of contrast material after placement of the third coil, we decided to initiate anticoagulation therapy to prevent thromboembolic complications.
How frequently does rupture of an aneurysm occur during endovascular aneurysm treatment? Overall, we observed intraprocedural aneurysm rupture in 3% of our patients, with a mortality rate of 20% and no long-term morbidity. The reported percentage of aneurysmal rupture that complicates embolization of cerebral aneurysms varies among studies. Viñuela et al (3) reported 11 cases of rupture, representing 2.7% of the treated aneurysms, among 403 acutely ruptured aneurysms that were treated at eight centers. This perforation rate is close to our results, to the 2% rate reported by McDougall et al (9), and to the data of a large metaanalysis of the treatment of intracranial aneurysms with coils conducted by Brilstra et al (6), who reported a perforation rate of 2.4% for ruptured aneurysms. In their preliminary experience, Guglielmi et al (22) observed five ruptures during the treatment of 127 aneurysms. Valavanis et al (10) reported a perforation rate of 4.2%, which is close to the 4.4% rate of perforation reported by Ricolfi et al (11) and to the data reported by Cognard et al (5), who observed six ruptures during endovascular treatment of 150 ruptured aneurysms. Raymond and Roy (4) reported six perforations of the aneurysm during 103 endovascular procedures for acutely ruptured aneurysms; these perforations resulted in no clinical deterioration in three cases but were responsible for procedure-related deaths in the other three cases. Five of these six perforations occurred during the authors' early experience; this finding indicates that increased experience seems to minimize incidence. Because five of the iatrogenic ruptures occurred in small aneurysms, the authors suggest that this complication could be minimized if acutely ruptured aneurysms smaller than 3 mm are not treated with GDCs (4). One might question why we treated our cases 1 and 3 by using an endovascular route, considering that these aneurysms were small (case 3) or had a relatively wide neck (case 1). However, on the basis of our overall experience, with good results achieved in more than 17 small aneurysms (2 and 3 mm) that were successfully treated with GDCs, and together with the neurosurgeon, we decided to treat these patients endovascularly. Ricolfi et al (11) also reported an increased fragility of smaller aneurysms and presented two possible explanations: 1) The surface area of the initial rupture is proportionally larger for small aneurysms than for large aneurysms, and 2) small coils that measure 2–3 mm in diameter have a higher shape memory and may therefore have a tendency to cause damage to the weakened site of initial rupture, as in our case 3. Additionally, the authors emphasize the role of immediate ventriculostomy as emergent management of rupture with severe SAH and intracranial hypertension during treatment. If necessary, we still recommend performing ventriculostomy before endovascular treatment, because the outcome after aneurysmal rupture during endovascular treatment is better in patients with ventriculostomy.
As seems to be the case with Raymond and Roy (4), one would expect that with increasing experience in endovascular aneurysm therapy, intraprocedural rupture would occur less frequently. In accordance with McDougall et al (9), we also observed aneurysm perforations during embolization after having treated more than 100 acutely ruptured aneurysms. Any interventional neuroradiologist who treats acutely ruptured aneurysms may face this complication, regardless of technical improvements and his or her skill level. Importantly, the interventional neuroradiologist should keep this worst-case scenario in mind and simulate crisis management before and during aneurysm embolization.
Conclusion
Aneurysmal rupture during endovascular GDC treatment of ruptured cerebral aneurysms is a rare event, but it remains a potential risk. It may be due to perforation with the microguidewire or microcatheter, or it may occur during coil placement.The clinical seriousness of the rupture may be variable, ranging from slight leakage of contrast material into the subarachnoid space to massive SAH with severe intracranial hypertension. Embolization of the aneurysm can be continued in most cases, and most patients who have treatment-related SAH survive without serious sequelae and with a better-than-expected outcome.
Footnotes
Address reprint requests to Arnd Doerfler, MD, Department of Neuroradiology, University of Essen Medical School, Hufelandstrasse 55, D-45122 Essen, Germany.
References
- 1.Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg 1974;41:125-145 [DOI] [PubMed] [Google Scholar]
- 2.Guglielmi G, Viñuela F, Sepetka I, Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach, I: electrochemical basis, technique, and experimental results. J Neurosurg 1991;75:1-7 [DOI] [PubMed] [Google Scholar]
- 3.Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg 1997;86:475-482 [DOI] [PubMed] [Google Scholar]
- 4.Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery 1997;41:1235-1246 [DOI] [PubMed] [Google Scholar]
- 5.Cognard C, Weill A, Castaings L, Rey A, Moret J. Intracranial berry aneurysms: angiographic and clinical results after endovascular treatment. Radiology 1998;206:499-510 [DOI] [PubMed] [Google Scholar]
- 6.Brilstra EH, Rinkel GJ, van der Graaf Y, van Rooij WJ, Algra A. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke 1999;30:470-476 [DOI] [PubMed] [Google Scholar]
- 7.McDougall C, Halbach VV, Dowd CF, Higashida RT, Larsen DW, Hieshima GB. Endovascular treatment of basilar tip aneurysms using electrolytically detachable coils. J Neurosurg 1996;84:393-399 [DOI] [PubMed] [Google Scholar]
- 8.Halbach VV, Higashida R, Dowd C, Barnwell SL, Hieshima GB. Management of vascular perforations that occur during neurointerventional procedures. AJNR Am J Neuroradiol 1991;12:319-327 [PMC free article] [PubMed] [Google Scholar]
- 9.McDougall C, Halbach VV, Dowd C, Higashida R, Larsen D, Hieshima G. Causes and management of aneurysmal hemorrhage occuring during embolization with Guglielmi detachable coils. J Neurosurg 1998;89:87-92 [DOI] [PubMed] [Google Scholar]
- 10.Valavanis A, Machado E, Chen JJ. Aneurysm rupture during GDC treatment: incidence, management and outcome. Neuroradiology 1996;38: (suppl 2) 45 [Google Scholar]
- 11.Ricolfi F, Le Guerinel C, Blustajn J, et al. Rupture during treatment of recently ruptured aneurysms with Guglielmi electrodetachable coils. AJNR Am J Neuroradiol 1998;19:1653-1658 [PMC free article] [PubMed] [Google Scholar]
- 12.Beamer YB, Corsino JF, Lynde RG. Rupture of aneurysm of the internal carotid artery during arteriography with filling of subarachnoid space and demonstration of a temporal lobe mass: case report. J Neurosurg 1969;31:224-226 [DOI] [PubMed] [Google Scholar]
- 13.Komiyama M, Tamura K, Nagata Y, Fu Y, Yagura H, Yasui T. Aneurysmal rupture during angiography. Neurosurgery 1993;33:798-803 [DOI] [PubMed] [Google Scholar]
- 14.Saitoh H, Hayakawa K, Nishimura K, et al. Rerupture of cerebral aneurysm during angiography. AJNR Am J Neuroradiol 1995;16:539-542 [PMC free article] [PubMed] [Google Scholar]
- 15.Lin JP, Kricheff II, Chaes NE. Blood pressure changes during retrograde brachial angiography. Radiology 1964;83:640-646 [DOI] [PubMed] [Google Scholar]
- 16.Sorimachi T, Takeuchi S, Koike T, Minakawa T, Tanaka R. Intra-aneurysmal pressure changes during angiography in coil embolization. Surg Neurol 1997;48:451-457 [DOI] [PubMed] [Google Scholar]
- 17.Batjer H, Samson D. Intraoperative aneurysmal rupture: incidence, outcome, and suggestions for surgical management. Neurosurgery 1986;18:701-707 [DOI] [PubMed] [Google Scholar]
- 18.Schramm J, Cedzich C. Outcome and management of intraoperative aneurysm rupture. Surg Neurol 1993;40:26-30 [DOI] [PubMed] [Google Scholar]
- 19.Houkin K, Kuroda S, Takahashi A, et al. Intra-operative premature rupture of cerebral aneurysms: analysis of the causes and management. Acta Neurochir (Wien) 1999;141:1255-1263 [DOI] [PubMed] [Google Scholar]
- 20.Willinsky R, terBrugge K. Use of a second microcatheter in the management of a perforation during endovascular treatment of a cerebral aneurysm. AJNR Am J Neuroradiol 2000;21:1537-1539 [PMC free article] [PubMed] [Google Scholar]
- 21.Qureshi AI, Luft AR, Sharma M, Guterman LR, Hopkins LN. Prevention and treatment of thromboembolic and ischemic complication associated with endovascular procedures, II: clinical aspects and recommendations. Neurosurgery 2000;46:1360-1376 [DOI] [PubMed] [Google Scholar]
- 22.Guglielmi G, Viñuela F, Duckwiler G. Endovascular treatment of posterior circulation aneurysms by electrothrombosis using electrically detachable coils. J Neurosurg 1992;77:515-524 [DOI] [PubMed] [Google Scholar]