Abstract
Summary: A 47-year-old woman, who had lost vision in her left eye because of a giant left supraclinoid internal carotid artery aneurysm, was referred for endovascular treatment. Parent-vessel occlusion was performed to obtain circulatory exclusion of the aneurysm. Eight days after treatment, she became hemiparetic and dysphasic. Repeat angiography showed compression of the left middle cerebral artery by the swelling giant aneurysm. Preventive measures should be taken to avert worsening of mass effect when giant aneurysms become thrombotic.
The goal of treatment of a giant intracranial aneurysm, either surgical or endovascular, is to prevent further aneurysmal rupture and to relieve mass effect. Endovascular treatment relies on various techniques, such as sac filling with coils, parent vessel occlusion (PVO), and sometimes flow reversal. We report a case of a giant aneurysm for which PVO led to delayed neurologic complications.
Case Report
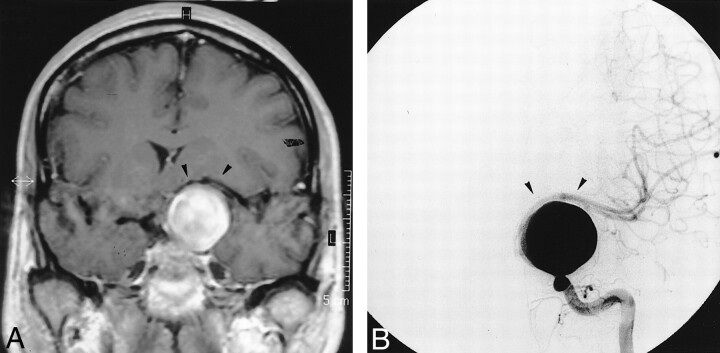
A 47-year-old woman was referred to our institution for assessment and possible treatment of a left supraclinoid internal carotid artery (ICA) aneurysm. The patient had a 7-mo history of progressive visual alteration in her left eye. Although a complete ophthalmologic examination was considered normal, MR imaging revealed a 27-mm-diameter left supraclinoid ICA aneurysm. It was noted that the dome of the aneurysm was in contact with the M1 portion of the left middle cerebral artery (MCA) (Fig 1A). The decision was made to angiographically study the patient and to endovascularly treat the aneurysm. Accordingly, the patient underwent full angiographic assessment that confirmed the diagnosis of a giant left supraclinoid ICA aneurysm (Fig 1B). It was decided that the most suitable treatment was PVO. While the patient was under neuroleptanalgesia (250 mg of IV administered aspirin) and full heparinization (a bolus of 5000 U followed by continuous IV injection at a rate of 3000 U/h), the left ICA was occluded with a detachable balloon (X-RAY no 3; Balt Extrusion, Montmorency, France) at the level of the ophthalmic artery. Control angiograms of the left common carotid, right ICA, and left vertebral arteries were obtained before balloon detachment and showed that the aneurysm was totally excluded, with no retrograde filling via the anterior communicating artery (ACoA), posterior communicating arteries (PCoAs), or left ophthalmic artery. The ACoA provided good flow to the left side of the brain (Fig 2), and no delay existed between the venous phases of both cerebral hemispheres on the right ICA control angiogram. After 30 min of occlusion, which the patient tolerated well according to continuous clinical neurologic testing, the balloon was detached. A second balloon was also placed upstream in the left ICA for added security.
fig 1.
MR image and angiogram obtained prior to treatment.
A, Coronal T1-weighted MR image after gadolinium chelate infusion shows the giant left supraclinoid aneurysm shifting the left MCA (arrowheads).
B, Left ICA angiogram (frontal projection) depicts the left giant nonthrombosed supraclinoid aneurysm stretching the left MCA (arrowheads).
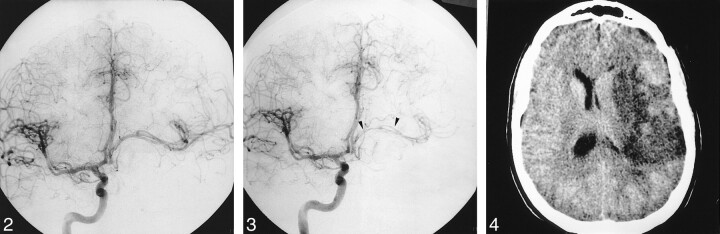
fig 2.
Right carotid angiogram (frontal projection), obtained immediately after left ICA occlusion, shows the absence of retrograde filling of the aneurysm and good cross flow via the ACoA.
fig 3. Right carotid angiogram (frontal projection, late arterial phase), obtained 8 d after left ICA occlusion, shows that the stretching and shifting of the left MCA had increased (arrowheads).
fig 4. Plain CT scan obtained 20 d after endovascular treatment shows a left MCA territory infarct.
During the first 24 h after the procedure, the patient had two transient episodes of dysphasia and right hemiplegia that resolved within a few minutes. The patient was treated with hypertensive and steroidal therapy and continued to undergo full heparinization to obtain an activated clotting time 2–2.5 times the baseline for 48 h. She was then switched to subcutaneous low-molecular-weight heparin at hypocoagulative doses, which was continued for 10 d. Additionally, she was prescribed oral aspirin (250 mg daily) for 3 wk. She was discharged from the hospital 5 d after the treatment, but returned 3 d later with progressive right hemiparesis and dysphasia. A CT scan obtained at this time suggested deep left temporal hypoattenuation, which was better seen 2 d later. No evidence of hemorrhage existed, and the aneurysm was thrombosed. Repeat angiography showed that the balloons were in a good position, and the aneurysm was still completely excluded from the circulation. However, blood flow in the M1 portion of the left MCA was even more attenuated than before the carotid occlusion, with very slow opacification of the distal branches of the MCA (Fig 3). Although revascularization via an extracranial-intracranial arterial bypass and surgical decompression of the aneurysm were considered, it was decided that the patient would be treated with full heparinization, hypertensive therapy, and steroids. Unfortunately, the patient did not improve and slowly deteriorated, despite increasing doses of IV inotropic drugs. After 4 d of medical treatment, her neurologic condition continued to deteriorate, manifested by a left third cranial nerve palsy, headaches, and vomiting. Another CT scan obtained at this time depicted a subarachnoid hemorrhage. Although the aneurysm was proximal to the PCoA, and this artery never filled the aneurysm, a third angiogram showed partial recanalization of the aneurysm, with retrograde filling via the PCoA. Anticoagulant therapy was stopped, and she was weaned from hypertensive therapy. A CT scan obtained 20 d after the carotid occlusion confirmed infarction of the deep territory of the MCA area (Fig 4). She remained hemiparetic and mildly dysphasic and was referred for rehabilitation. After discharge, her motor deficit partially improved, and her dysphasia had improved at 2-y follow-up.
Discussion
Endovascular treatment of giant intracranial aneurysms with coils alone has been shown to be ineffective long- term because of coil compaction (1). However, decreasing aneurysmal pulsatility with circulatory exclusion of the aneurysm from the circulation and some shrinkage of the sac are thought to occur and to be sufficient to alleviate the compressive effect of the aneurysm (2–7). Unfortunately, worsening of the mass effect can happen shortly after treatment, leading to compression of surrounding parenchyma, especially ocular tracts or cranial nerves (8–10). Delayed ischemic events after PVO may be related to insufficient cross flow, a thromboembolic phenomenon from the occlusion site. In our case, we believe that thrombosis of the aneurysm resulted in swelling, with further compression of the M1 portion of the left MCA, as suggested by the delayed and progressive onset of the deficit. Repeat angiography showed a severe narrowing of the M1 portion of the MCA in contact with the aneurysmal dome; the cross flow through the ACoA was not impaired.
Surgical decompression of the aneurysmal sac might have been considered, although surgery for giant aneurysm is hazardous. Preventive bypass might also have been advocated, but would not have been satisfactory to avoid infarction in the lenticulostriate arteries arising from the compressed M1 segment. Experiences, both neurosurgical and endovascular, have shown that giant aneurysms that are partially or not thrombotic can be particularly susceptible to swelling complications (3, 11). For some authors, the severity of aggravation after PVO is directly proportional to the size of the remnant lumen and to the clot that may result after thrombosis of the aneurysm (3). This may be linked to the release of vasoactive factors, such as vascular endothelial growth factor (VEGF) released during platelet aggregation, which are known to play an active role in angiogenesis, endothelial growth, and endothelial permeability (12). Corticosteroids can decrease the release of VEGF. However, pretreatment with steroids would have, theoretically, decreased the mass effect in the patient described herein. As partial embolization with coils prior to PVO would have decreased the volume of the circulating sac, perhaps it would have reduced the severity of thrombosis, swelling, and mass effect after PVO. On the basis of this case, we now prefer to purposely undercoil giant aneurysms and perform a delayed PVO a few weeks later in order to decrease the risk of acute aneurysmal thrombosis.
Footnotes
Address reprint requests to Jacques Moret, MD, Service de Neuroradiologie Interventionnelle, Hôpital de la Fondation Rothschild, 25-29 rue Manin, 75940 Paris cedex 19, France.
References
- 1.Gobin YP, Vinuela F, Gurian JH, et al. Treatment of large and giant fusiform intracranial aneurysms with Guglielmi detachable coils. J Neurosurg 1996;84:55-62 [DOI] [PubMed] [Google Scholar]
- 2.Drake CG. Giant intracranial aneurysms: experience with surgical treatment in 174 patients. Clin Neurosurg 1979;26:12-95 [DOI] [PubMed] [Google Scholar]
- 3.Halbach VV, Higashida RT, Dowd CF, et al. The efficacy of endosaccular aneurysm occlusion in alleviating neurological deficits produced by mass effect [see comments]. J Neurosurg 1994;80:659-666 [DOI] [PubMed] [Google Scholar]
- 4.Hirasawa T, Tsubokawa T, Katayama Y, et al. Growth of a giant aneurysm following complete thrombosis by detachable balloon occlusion. Surg Neurol 1992;38:283-286 [DOI] [PubMed] [Google Scholar]
- 5.Larson JJ, Tew JM Jr, Tomsick TA, van Loveren HR. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery 1995;36:26-30 [PubMed] [Google Scholar]
- 6.Malisch TW, Guglielmi G, Vinuela F, et al. Intracranial aneurysms treated with the Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg 1997;87:176-183 [DOI] [PubMed] [Google Scholar]
- 7.Malisch TW, Guglielmi G, Vinuela F, et al. Unruptured aneurysms presenting with mass effect symptoms: response to endosaccular treatment with Guglielmi detachable coils: Part I, symptoms of cranial nerve dysfunction. J Neurosurg 1998;89:956-961 [DOI] [PubMed] [Google Scholar]
- 8.Hecht ST, Horton JA, Yonas H. Growth of a thrombosed giant vertebral artery aneurysm after parent artery occlusion. AJNR Am J Neuroradiol 1991;12:449-451 [PMC free article] [PubMed] [Google Scholar]
- 9.Strother CM, Eldevik P, Kikuchi Y, Graves V, Partington C, Merlis A. Thrombus formation and structure and the evolution of mass effect in intracranial aneurysms treated by balloon embolization: emphasis on MR findings. AJNR Am J Neuroradiol 1989;10:787-796 [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki N. Partially thrombosed aneurysm presenting as the sudden onset of bitemporal hemianopsia. Neurosurgery 1988;22:564-566 [DOI] [PubMed] [Google Scholar]
- 11.Whittle IR, Williams DB, Halmagyi GM, Besser M. Spontaneous thrombosis of a giant intracranial aneurysm and ipsilateral internal carotid artery: case report. J Neurosurg 1982;56:287-289 [DOI] [PubMed] [Google Scholar]
- 12.Maloney JP, Silliman CC, Ambruso DR, Wang J, Tuder RM, Voelkel NF. In vitro release of vascular endothelial growth factor during platelet aggregation. Am J Physiol 1998;275:H1054-H1061 [DOI] [PubMed] [Google Scholar]