Abstract
BACKGROUND AND PURPOSE: Gamma knife radiosurgery is an alternative for the treatment of medically refractory trigeminal neuralgia. Few reports of posttreatment MR imaging appearance of cranial nerve V exist. Our purpose was to define MR imaging characteristics in cranial nerve V after gamma knife radiosurgery.
METHODS: We retrospectively reviewed MR images of 15 patients who underwent gamma knife radiosurgery for trigeminal neuralgia. Radiation doses were 35–45 Gy at the 50% isodose line. Thin-section T2-weighted images and contrast-enhanced and nonenhanced T1-weighted images were obtained the day of radiosurgery and within the next 5 mo. Images were scored for enhancement and hyperintensity on T2-weighted images. Time to follow-up imaging and radiation dose were recorded.
RESULTS: Mean time to follow-up imaging was 61 ± 29 d. Posttreatment T2-weighted images showed stable signal intensity in all cases, with radiosurgical target site enhancement in 10. All five patients whose images did not show treatment-related enhancement received radiation doses of 35 Gy. The data suggested a correlation between enhancement with radiation dose (P = .06). No correlation of enhancement with treatment response or time to follow-up existed (P > .05).
CONCLUSION: The trigeminal nerve often enhances at the target site after radiosurgery. Lack of trigeminal nerve enhancement occurred only with lower doses (35 Gy at 50%). MR imaging may be useful to confirm the presence and location of the treatment site after gamma knife radiosurgery for trigeminal neuralgia.
Trigeminal neuralgia is a paroxysmal pain syndrome corresponding to the distribution of cranial nerve V. Patients have severe lancinating pain, with minimal external stimulation of the affected dermatome. In severe cases, patients can experience malnutrition and weight loss due to an inability to eat. Fortunately, primary drug therapy is frequently effective in controlling or eliminating the symptoms of this disease. For patients with medically refractory disease or intolerable adverse effects from long-term medical therapy, several surgical options are available. These include glycerol rhizolysis, thermal rhizotomy, and peripheral nerve blocks (1–3). More effective surgical options are more invasive and include microvascular decompression (4).
Recently, the treatment of medically refractory trigeminal neuralgia with gamma knife radiosurgery has been evaluated. One large series had excellent responses (pain relief without the need for continued medical therapy) in 72% of treated patients, good responses (≥50% reduction in pain, with or without low-dose medication) in 10% of treated patients, and failure in 18% of treated patients after 1.5-y follow-up (5). Results have varied, however, and other investigators report different response rates (6–10).
The differences in reported response rates of trigeminal neuralgia to radiosurgery have been attributed to multiple factors. These include differences in previous invasive treatment, dose delivery, and target location during radiosurgery, as well as differences in imaging technique before treatment planning. Previous reports (5, 11, 12) suggest that inaccurate targeting due to distortion of the MR images may contribute to this variable response rate, whereas others (13) disagree. For patients with poor clinical response, confirmation of target tissue change with MR imaging may be useful in verifying accurate targeting at the time of treatment. Previous reports suggest that cranial nerve V may enhance after gamma knife radiosurgery, although others found no MR imaging signal intensity changes (5, 14). We hypothesized that the effects of radiosurgery on cranial nerve V may include signal intensity changes within the nerve on MR images. The purpose of this pilot study was to identify and characterize the type of MR signal intensity changes in cranial nerve V that result from gamma knife treatment of medically refractory trigeminal neuralgia.
Methods
Patients
Gamma knife radiosurgery was performed in 15 patients between December 1998 and June 2000 for the treatment of medically refractory trigeminal neuralgia. Seven men and eight women were treated. Patient ages ranged from 27 to 88 y (mean age, 69 ± 16 y). Each patient had a history of trigeminal neuralgia without atypical features. Patients with cluster headache, atypical facial pain, and trigeminal neuralgia in association with multiple sclerosis or tumors were excluded from the analysis. Patients with MR images showing abnormal signal intensity or enhancement in cranial nerve V before radiosurgery were also excluded. All patients received therapy with carbamazepine and at least one other anticonvulsant medication, which had failed. Institutional review board approval was obtained for review of existing data. Patient informed consent was not required.
MR Imaging
All MR examinations were performed with the same 1.5-T system (Signa horizon 5.8; GE Medical Systems, Milwaukee, WI) before and within 5 mo after gamma knife radiosurgery. Imaging consisted of T1-weighted localizing with T1-weighted and fast spin-echo T2-weighted axial and coronal imaging of cranial nerve V. For axial T1-weighted images, the parameters were 400/11/2 (TR/TE/excitations); matrix, 256 × 192; field of view, 15 cm; section thickness, 1 or 2 mm; and no section gap. For axial fast spin-echo T2-weighted images, the parameters were 3600/85/3; matrix, 256 × 192; field of view, 16 cm; section thickness, 1.5 or 2 mm; and no section gap. Coronal images were obtained from the middle of the pons to the sella turcica with the above parameters with a 3-mm thickness and a 1-mm gap. Acquisition of T1-weighted images were repeated after the IV administration of gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ), to a total of 0.2 mL/kg. A flow compensation pulse was added to the contrast-enhanced imaging sequence. In the gamma knife planning study, additional T1-weighted images were obtained, with a field of view of 25 cm to accommodate the fiducial markers required for the radiosurgical planning system.
Radiosurgery
Radiosurgery was performed with a 201 source, cobalt 60 gamma system (Gamma Knife; Elekta Instrument, Stockholm, Sweden). Patients were treated with either one or two isocenters, by using a 4-mm collimator. A dose range of 35–45 Gy at the 50% isodose line was used. The retrogasserian portion of the nerve, corresponding to the unmyelinated dorsal root entry zone, was targeted in each case. The coordinates of the isocenters were chosen to bring the 30% isodose line to the edge of the pons. No corticosteroids were used at the time of treatment or during the ensuing 6 wk.
Image Review
For review, all images were reprinted with identical window and/or level settings and printing format. A neuroradiologist (R.A.A.) with a Certificate of Added Qualification who was unaware of the side of treatment and treatment response reviewed all MR images. Images were scored on a qualitative three-point scale based on visually perceived differences in signal intensity. The signal intensity of cranial nerve V was compared with that on the pretreatment images, as well as with that of the contralateral side and on nonenhanced images. Images were scored on a three-point scale for contrast enhancement and abnormal signal intensity on T2-weighted images of cranial nerve V. A score of 1 represented no change in signal intensity or enhancement compared with that of the pretreatment images or contralateral nerve; a score of 2 represented subtle change in signal intensity or enhancement compared with that of pretreatment images, which was more obvious when compared with the contralateral side; and a score of 3 represented obvious focal change in signal intensity or enhancement compared with that of both the pretreatment images and contralateral side. All perceived signal intensity differences had to be present in two imaging planes or else a score of 1 was assigned. Signal intensity changes were then compared with radiosurgical target location by reviewing the radiosurgical treatment plan. The distance of the center of the area of abnormal signal intensity in cranial nerve V and its length along the nerve was measured by using a 3D workstation (Advantage Windows 3.1; GE Medical Systems) and recorded for all cases with abnormal signal intensity. At a separate time, the distance of the center of the target site from the pontine edge was also measured by using the 3D workstation. All measurements of the target site center were determined by consensus of the neuroradiologist and neurosurgeon (R.A.F.), who were blinded to the distance measurements obtained during image review.
Statistics
The distance and length of the area with abnormal signal intensity from the pontine edge along each treated nerve was compared with the distance of the target site center from the pontine edge. Correlation of the target center location with the area of enhancement was considered positive if the target center was located within the confines of the areas of abnormal signal intensity along the nerve.
The clinical response of the patient was graded on a four-point scale, with excellent response defined as reduction in pain of 90% or more without the need for medical therapy; good response, 50%–90% reduction in pain with reduced medication dose; fair response,10%–50% reduction in pain with full pretreatment medication dose; and poor response, 0%–10% reduction in pain with full pretreatment medication dose. Treatment dose and time to follow-up MR imaging were also recorded for each patient. The two-tailed Barnard unconditional test for the difference between two binomial proportions (CYTEL Software, Cambridge, MA) (15) was used to compare clinical response, treatment dose, and time to follow-up imaging data.
Results
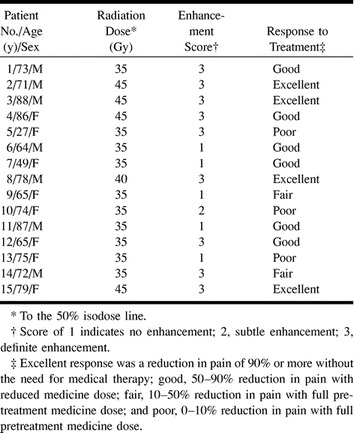
Fifteen patients were examined with contrast-enhanced MR imaging performed on the day of radiosurgery and within the ensuing 5 mo. All patients except one were treated with a single isocenter. Ten patients were treated with 35 Gy at the 50% isodose line, and five were treated with 45 Gy at the 50% isodose line. The mean time to follow-up MR imaging was 61 ± 29 d. Enhancement of cranial nerve V was seen as early as 32 d and as late as 188 d after treatment. No patient underwent imaging beyond 188 d after treatment. No signal intensity abnormalities of cranial nerve V were depicted on T2-weighted images in any of the patients before or after treatment. No patient had abnormal enhancement of cranial nerve V before treatment. Ten patients had focal enhancement of cranial nerve V at posttreatment follow-up. Nine of 10 enhancing cranial nerves were given qualitative scores of 3 (Fig 1); one received a score of 2 (Fig 2). All patients treated with 45 Gy to the 50% isodose line had cranial nerve V enhancement. Half of the patients treated with 35 Gy to the 50% isodose line had enhancement of cranial nerve V (Table). The two-tailed Barnard unconditional difference between the high- and low-dose treatment groups regarding contrast enhancement revealed a P value of .06. This finding strongly suggests that the higher radiation dose resulted in a higher proportion of enhancement than did the lower radiation dose, but the conclusion was not statistically significant with the commonly accepted criterion. No statistically significant correlation of the follow-up interval (P > .05) with enhancement was present in this small sample.
fig 1.
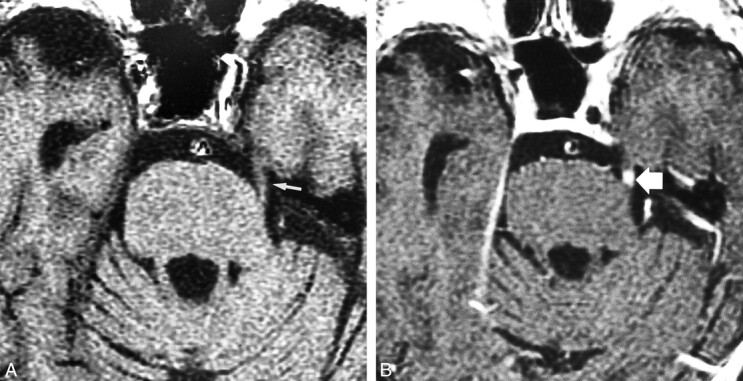
Nine of 10 enhancing cranial nerves had a qualitative score of 3.
A, Nonenhanced T1-weighted MR image (400/11/2) in an 88-year-old man with trigeminal neuralgia on the left, obtained 78 d after gamma knife radiosurgery. An excellent response to treatment was achieved. Treatment dose was 45 Gy to the 50% isodose line. Note the intensity of cranial nerve V (arrow).
B, Contrast-enhanced T1-weighted MR image (400/11/2) of cranial nerve V shows marked (grade 3) focal enhancement of the nerve (arrow) adjacent to the pontine edge.
fig 2.
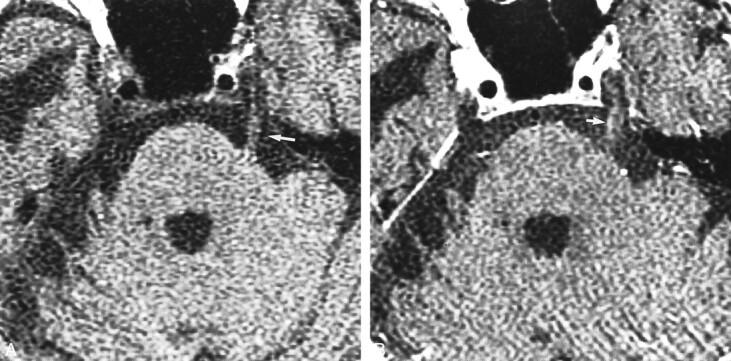
One of 10 enhancing cranial nerves had a qualitative score of 2.
A, Nonenhanced T1-weighted MR image (400/11/2) in a 74-year-old woman with trigeminal neuralgia, obtained 54 d after treatment with gamma knife radiosurgery. Treatment resulted in a poor response. Treatment dose was 35 Gy to the 50% isodose line. Cranial nerve V (arrow) is well depicted.
B, Contrast-enhanced T1-weighted MR image (400/11/2) shows subtle (grade 2) enhancement of cranial nerve V (arrow).
TABLE 1:
Patients with trigeminal neuralgia treated with gamma knife radiosurgery
In 10 of 10 cases that had enhancement after radiosurgery, the gamma knife target site center was within the enhancing portion of the nerve. The length of enhancement along the nerve ranged from 3.3 to 9.4 mm. The 9.4-mm length occurred in the only case treated with two isocenters. The average length of enhancement was 4.9 ± 2.1 mm.
Twelve of 15 patients had fair, good, or excellent clinical responses at last follow-up; clinical failures occurred in three, two of whom required microvascular decompression (with good postoperative pain relief in both cases). Enhancement of cranial nerve V at the target site occurred in one of three patients with clinical treatment failure and in eight of 12 patients with other clinical responses. No statistically significant correlation between clinical response and enhancement existed in this small sample (P > .05).
Discussion
These findings suggest that changes in the imaging characteristics in cranial nerve V occur after treatment with gamma knife radiosurgery and consist of focal enhancement of the nerve at the site of the radiosurgical target, seen as early as 32 d and as late as 188 d after treatment. Enhancement was detected in 10 of 15 patients. Although no statistically significant correlation of treatment dose and enhancement was found in this small sample, the P value of .06 strongly suggests that treatment dose may have an effect on postprocedural enhancement. All nerves treated with higher dose showed enhancement, but only 50% of those treated with the lower dose showed enhancement. Also, a previous report (14) of contrast-enhanced MR imaging in 14 patients treated with 35 Gy to the 50% isodose line failed to show enhancement at the target site in any of the patients. Perhaps with a larger sample, a correlation of dose and enhancement will emerge.
The detection of enhancement in cranial nerve V after radiosurgery has several potential uses. Enhancement serves as a confirmation that the target was accurately treated in patients with a suboptimal clinical response. This information may be useful in planning the next course of treatment or in target placement at repeat gamma knife treatment. Contrast enhancement of the original treatment site can help the neurosurgeon position new targets, either in contiguous or consistent locations relative to that of the previous target. In all cases with enhancement in this study, the target site, as determined by reviewing the gamma knife treatment plan, was located within the area of enhancement. This method provides in vivo quality assurance for use in patients with nerves that enhance. Finally, exact target location relative to the pontine edge and along the retrogasserian portion of cranial nerve V may be a factor in treatment success (5–7). The potential for correlation of imaging changes in cranial nerve V with treatment response and/or complications can make future research efforts more meaningful and can potentially refine current target selection criteria.
For patients without enhancement of cranial nerve V after gamma knife radiosurgery, the situation is more ambiguous. For those with a good clinical result, it may be assumed that the target was accurately treated. For those without a good clinical result, lack of enhancement suggests that the target was not accurately treated, especially if relatively high doses were used (45 Gy to the 50% isodose line). This finding may prompt review of the target position and the quality of the treatment planning study. If nerve visualization was suboptimal, repeat treatment in this population with more extensive treatment planning, perhaps with complementary CT or CT cisternography, may be appropriate.
Conclusion
We conclude that MR imaging with contrast enhancement results in enhancement of the target site after gamma knife radiosurgery in a proportion of patients. When enhancement occurs, its presence in the nerve strongly correlates with the gamma knife target site. Although no statistically significant correlation of treatment dose or clinical response and enhancement was found, the data strongly suggest that a correlation with treatment dose exists. Contrast-enhanced MR imaging may be useful in eliminating the missed target as a cause in some patients with failed gamma knife radiosurgery of medically refractory trigeminal neuralgia.
Acknowledgments
We thank William Greco, PhD, MBA, Director of Biomathematics/Biostatistics, Roswell Park Cancer Institute, Core Grant, California, for help with the statistical analysis of the data.
Footnotes
Address reprint requests to Ronald A. Alberico, MD, Director of Neuroradiology, Department of Diagnostic Radiology, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263.
References
- 1.Jacob RP, Rhoton AL Jr. Diagnosis and non-operative management of trigeminal neuralgia. In: Youmans JR, ed. Neurological Surgery. Philadelphia, Pa: W.B. Saunders; 1996:3376–3385
- 2.Lunsford LD. Trigeminal neuralgia: treatment by glycerol rhizotomy. In: Wilkins RH, Rangachary SS, eds. Neurosurgery. New York, NY: McGraw-Hill; 1996:3953–3959
- 3.Broggi G, Franzini A, Lasio G, Giorgi C, Servello D. Long-term results of percutaneous retrogasserian thermorhizotomy for “essential” trigeminal neuralgia: considerations in 1000 consecutive patients. Neurosurgery 1990;26:783-787 [DOI] [PubMed] [Google Scholar]
- 4.Janetta PJ. Trigeminal neuralgia: treatment by microvascular decompression. In: Wilkins RH, Rengachary SS, eds. Neurosurgery. New York, NY: McGraw-Hill; 1996:3961–3968
- 5.Young RF, Vermeulen SS, Grimm P, Blasko J, Posewitz A. Gamma knife radiosurgery for treatment of trigeminal neuralgia: idiopathic and tumor related. Neurology 1997;48:608-614 [DOI] [PubMed] [Google Scholar]
- 6.Urgosik D, Vymazal J, Vladyka V, Liscak R. Gamma knife treatment of trigeminal neuralgia: clinical and electrophysiological study. Stereotact Funct Neurosurg 1998;70: (suppl 1) 200-209 [DOI] [PubMed] [Google Scholar]
- 7.Kondziolka D, Lunsford LD, Flickinger JC. Gamma knife radiosurgery as the first surgery for trigeminal neuralgia. Stereotact Funct Neurosurg 1998;70: (suppl 1) 187-191 [DOI] [PubMed] [Google Scholar]
- 8.Rand RW, Jacques DB, Melbye RW, Copcutt BG, Levenick MN, Fisher MR. Leksell gamma knife treatment of tic douloureux. Stereotact Funct Neurosurg 1993;61: (suppl 1) 93-202 [DOI] [PubMed] [Google Scholar]
- 9.Regis J, Manera L, Dufour H, Porcheron D, Sedan R, Peragut JC. Effect of the gamma knife on trigeminal neuralgia. Stereotact Funct Neurosurg 1995;64: (suppl 1) 182-192 [DOI] [PubMed] [Google Scholar]
- 10.Kondziolka D, Perez B, Flickinger JC, Habeck M, Lunsford LD. Gamma knife radiosurgery for trigeminal neuralgia. Arch Neurol 1998;55:1524-1529 [DOI] [PubMed] [Google Scholar]
- 11.Cohen DS, Lustgarten JH, Miller E, Khandji AG, Goodman RR. Effects of coregistration of MR to CT images on MR stereotactic accuracy. J Neurosurg 1995;82:772-779 [DOI] [PubMed] [Google Scholar]
- 12.Kondziolka D, Dempsey PK, Lundsford LD, et al. A comparison between magnetic resonance imaging and computed tomography for stereotactic coordinate determination. Neurosurgery 1992;30:402-407 [DOI] [PubMed] [Google Scholar]
- 13.Bednarz G, Downes MB, Corn BW, Curran WJ, Goldman HW. Evaluation of the spatial accuracy of magnetic resonance imaging-based stereotactic target localization for gamma knife radiosurgery of functional disorders. Neurosurgery 1999;45:1156-1163 [DOI] [PubMed] [Google Scholar]
- 14.Morales RE, Friedman DP, Goldman HW. Role of MR imaging in the follow-up of patients with medically refractory trigeminal neuralgia undergoing stereotactic radiosurgery using gamma knife [abstr]. Proc ASNR/ASHNR 1999;May 23:58 [DOI] [PubMed]
- 15.Suissa S, Shuster J. Exact unconditional sample sizes for the 2 × 2 binomial trial. J R Statist Soc Ser A 1985;148:317-327 [Google Scholar]


