Until the advent of MR imaging, knowledge of the structure of myelin and the process of myelination were of little importance to the neuroradiologist. Other than some mild changes in the attenuation of white matter, myelination resulted in no significant alterations of CT (1) or sonographic studies. MR studies, on the other hand, have been increasingly used for pediatric brain imaging. MR imaging's greater sensitivity to small changes in the water content of brain tissue, to changes in the binding of free water (revealed by magnetization transfer), and to the extent and anisotropy of water diffusion (revealed by diffusion imaging) has cast new light on this very complex and important molecule. Assessing myelination has become a key component of evaluating the child with delayed development. Moreover, better understanding of the nature of myelin and the effect of its different components on MR imaging parameters may help us to understand and diagnose inborn errors of metabolism better. In this review, I discuss what is known regarding the function and structure of CNS myelin and the effects of the various components of myelin on the signal imparted to the MR image.
Myelin Function
The myelin sheath is an extended, modified plasma membrane that is wrapped in a spiral fashion around a portion of an axon. Each myelin sheath is composed of multiple segments of myelin, which are modified extensions of oligodendroglial cell processes. Each oligodendrocyte can contribute myelin to as many as 50 different axons. The sections of myelin are separated from each other by small segments in which the bare axon is exposed to the interstitial space. These segments, called nodes of Ranvier, are the location of multiple sodium channels. When the axon membrane is excited, the generated electrical impulse cannot flow through the high-resistance myelin sheath and therefore flows out through and depolarizes the axonal membrane at the next node, which might be 1 mm or farther away. The low capacitance of the sheath allows depolarization of the remaining membrane between the nodes with little energy and markedly increased speed. The functional advantage rendered by myelination is illustrated by comparison of two nerve fibers that both conduct impulses at a speed of 25 m/s at 20°C; the unmyelinated giant axon of the squid (500 μm in diameter) requires 5000 times more energy and occupies 1500 times more space than does a 12-μm myelinated axon in a frog (2). In addition to its importance in nerve impulse conduction, myelin may have a symbiotic relationship with the axon. Evidence has also been published suggesting that myelin is synergistic with the developing axon and that the axonal cytoskeleton does not form properly in the absence of myelin (3).
Myelin Structure
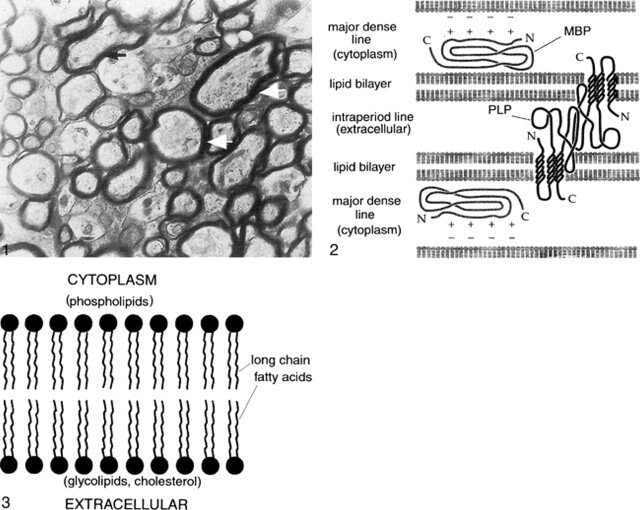
Examination of myelin under an electron microscope shows that the sheath is composed of multiple layers that are wound radially around the long axis of the axon (Fig 1). Examination with polarized microscopy and radiographic diffraction has shown that the layers have a protein-lipid-protein-lipid-protein structure (2, 4). Chemical investigations have determined that the lipid layers are in the cell membrane, composed of a bimolecular layer of hydrocarbon chains, cholesterol, phospholipids, and glycolipids. A highly electron-dense line, called the major period line, contains myelin basic protein (MBP), an intracellular protein attached to the inner surface of the cell membrane and situated mainly in the cytoplasm (Fig 2). A less electron-dense protein line is called the intraperiod line; it represents proteolipid protein (PLP) in the outer portion of the cell membrane and in the extracellular space (Fig 2).
fig 1.
Electron micrograph of myelinated axons in the optic nerve of a 3-week-old rat. Myelin wraps around the axon in multiple spirals to speed transmission of action potentials. Arrows point to the thickest spirals, which are around the largest axons.fig 2. Schematic of the structure of myelin. Myelin is composed of multiple layers having a protein-lipid-protein-lipid-protein structure. The lipid layers are in the cell membrane, composed of a bimolecular layer of hydrocarbon chains, cholesterol, phospholipids, and glycolipids. A highly electron-dense line, the major period line, contains MBP, an intracellular protein attached to the inner surface of the cell membrane and situated mainly in the cytoplasm. A less electron-dense protein line, the intraperiod line, represents PLP in the outer portion of the cell membrane and in the extracellular space. PLP interacts homophilically with similar PLP chains from the surface of the myelin membrane in the next loop of the spiral. In addition, the lipophilic amino acid tryptophan (not shown) is present on the outer surface edge of the PLP and may interact with galactocerebrosides in the outer lipid membrane of the adjacent myelin spiral. MBP forms dimers (chemical bonds with other MBPs) within the cytoplasm of the myelin sheath. MBP is thought to stabilize the myelin spiral at the major dense line by interacting with negatively charged lipids at the cytoplasmic surface of the lipid membrane.fig 3. Close-up schematic of myelin lipid bilayer. The lipid layer of myelin is composed of cholesterol, phospholipid, and glycolipid in an approximately 4:3:2 ratio for adult CNS myelin. It appears that most of the glycolipid (in the form of galactocerebroside and sulfatide) and cholesterol are in the outer layer of the membrane, exposed to the extracellular space. In contrast, the phospholipids, of which ethanolamine-containing plasmalogen is the most abundant type, are hydrophobic and are located exclusively in the inner (cytoplasm) side of the membrane. The area between the outer and inner membrane layers is composed primarily of hydrocarbon chains (long chain fatty acids)
Some knowledge had been gained regarding the structure of the proteins and lipid layers of myelin. This structure is of some importance in understanding the MR imaging characteristics of myelin and will therefore be discussed in some detail. The lipid layer of myelin is composed of cholesterol, phospholipid, and glycolipid in an approximately 4:3:2 ratio for adult CNS myelin. It appears that most of the glycolipid (in the form of galactocerebroside and sulfatide) and cholesterol is in the outer layer of the membrane, exposed to the extracellular space. Both glycolipids and cholesterol have functional groups that interact strongly with water; as discussed in the next section, this interaction is important in the MR characteristics of brain myelination. In contrast, the phospholipids, of which ethanolamine-containing plasmalogen is the most abundant type, are hydrophobic and are located exclusively in the inner (cytoplasm) side of the membrane (Fig 3) (5). The area between the outer- and inner-membrane layers is composed primarily of hydrocarbon chains (long chain fatty acids) (Fig 3). At least 50% of these hydrocarbons contain one or more double bonds, which result in bends or kinks in these otherwise straight chains (6). The bends and kinks prevent tight packing of the lipid molecules, allowing them to remain in a fluid state; if too closely packed, they would crystallize. (It is thought that cholesterol also helps to keep the membrane bilayer from crystallizing by interceding between some of the hydrocarbon chains [6]). However, the bends and kinks in the hydrocarbon chains also render the membrane less thermodynamically stable. Too much variation in the lengths of the hydrocarbon chains or too many double bonds reduce the stability to the point at which the membrane is more susceptible to breakdown by normal physiological processes (7, 8).
The two major structural proteins of myelin are PLP and MBP. PLP constitutes approximately 50% by weight of myelin proteins (2, 6, 9). It is composed of four helices that span the lipid membrane and extend into the extracellular space as molecular chains that are well suited for homophilic interaction with similar PLP chains from the surface of the myelin membrane in the next loop of the spiral (Fig 2) (10). This homophilic interaction leads to close apposition of outer membranes of adjacent myelin spirals; the accumulation of electron dense PLP in this space results in the appearance of the intermediate dense line (intraperiod line) as revealed by electron microscopy (11, 12). The lipophilic amino acid tryptophan is present on the outer surface edge of the PLP and may interact with galactocerebrosides in the outer lipid membrane of the adjacent myelin spiral, providing the compacted myelin with increased stability (13). When either PLP or galactocerebrosides are altered by mutation, myelin is unstable and slowly degenerates, forming large vacuoles that split along the intraperiod line (13). In addition, some evidence has been published indicating that PLP plays a metabolic role in maintaining axonal metabolism (11).
The MBPs are a group of isoforms (chemical compounds with the same molecular structural components but with the components arranged differently) that constitute 30% to 40% by weight of myelin proteins (2, 6, 9). MBP is located on the cytoplasmic face of the myelin membrane. It is an electron-dense compound and is thought to be the major component of the major dense line that is revealed by electron microscopy. Some evidence suggests that MBP forms dimers (ie, it forms chemical bonds with other MBPs within the cytoplasm of the myelin sheath) (2). MBP is thought to stabilize the myelin spiral at the major dense line by interacting with negatively charged lipids at the cytoplasmic surface of the lipid membrane (MBP has a positive charge) (Fig 2) (2). Other functions, if any, are unknown.
A few other proteins have been identified in myelin, and more are being identified continually (14). Myelin-associated glycoprotein (MAG) is important in the development of the myelin sheath. It is the major mediator of the axonal-glial contacts that are essential for the initiation of myelination (15, 16). Another important function for MAG is as scaffolding, keeping the folds of immature myelin (or oligodendroglial cell processes) at a regular distance apart while the major structural proteins are being formed (17, 18). MAG also plays a role in axonal guidance because it is the component of myelin that represses growth of neurites (axons and dendrites) (19). However, MAG is ultimately excluded as a major structural component of the mature, compact myelin sheath. An enzyme, 2,3-cyclic nucleotide 3-phosphodiesterase comprises up to 5% of myelin protein, but its function is unknown. Similarly, the functions of myelin oligodendrocyte glycoprotein and oligodendrocyte-myelin glycoprotein are unknown (2, 14).
MR Imaging of Myelin in Development and Disease
Development
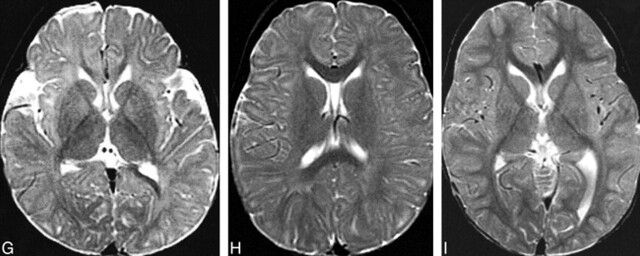
It is well known that MR imaging allows an assessment of the state of maturation (and thus myelination) of the child's brain (20–29). Maturation of the brain is associated with shortening of T1 and T2 relaxation times (24), reduced water diffusion (30, 31), increased diffusion anisotropy (32), and increased magnetization transfer (33). These changes in MR imaging parameters are thought to be largely the result of myelination, and thus, they can be used to assess whether brain maturation is progressing normally. As the white matter myelinates, it changes from hypointense to hyperintense relative to gray matter on T1-weighted images and from hyperintense to hypointense relative to gray matter on T2-weighted images (Fig 4). Specific milestones have been established for times when the changes in white matter intensity (relative to gray matter intensity) can be seen (20–23, 26–28). Magnetization transfer tends to increase with brain myelination, both topologically (Fig 5) and quantitatively (D. Enzmann, personal communication). However, no milestones have yet been published for changes in apparent diffusion coefficients (ADCs), degrees of anisotropy, or magnetization transfer ratios in different portions of the brain during infancy and childhood.
fig 4.
MR changes of myelination at the level of the basal ganglia.
A and B, Spin-echo (600/11 [TR/TE]) image (A) of a neonate shows that myelination (white matter with short T1 [hyperintensity on T1-weighted images] and short T2 [hypointensity on T2-weighted images]) is limited to the posterior limb of the internal capsule at this level. Spin-echo (3000/120) image (B) of the same patient shown in panel A.
C and D, Spin-echo (600/11) image (C) of a 5-month-old patient shows hyperintensity in the entire internal capsule, optic radiations, and splenium of the corpus callosum. Spin-echo (3000/120) image (D) of the same patient shown in panel C shows hypointensity limited to the posterior limb of the internal capsule and a portion of the optic radiations.
E and F, Spin-echo (600/11) image (E) of an 8-month-old patient shows hyperintensity in all white matter except the immediate subcortical regions. Spin-echo (3000/120) image (F) of the same patient shown in panel E shows hypointensity in the entire corpus callosum, the entire posterior limb of the internal capsule, and part of the anterior limb of the internal capsule.
G, Spin-echo (2500/80) image of a 12-month-old patient shows hypointensity in the entire internal capsule, in the subcortical white matter of the motor cortex, and in the subcortical white matter of the visual cortex.
H, Spin-echo (2500/80) image of an 18-month-old patient shows hypointensity in most of the deep white matter but lack of maturity of subcortical white matter.
I, Image of a 24-month-old patient shows that essentially all white matter is hypointense.
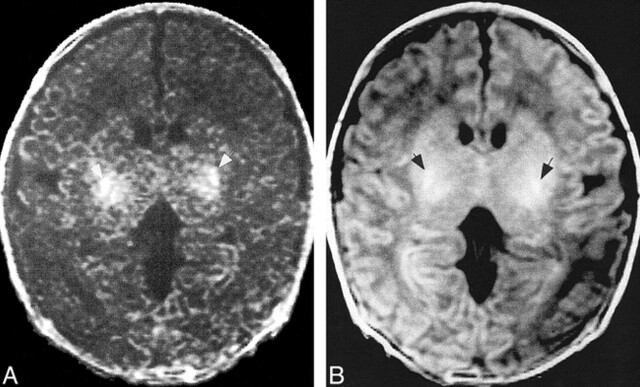
fig 5.
Images of a neonate.
A, Magnetization transfer image shows that magnetization transfer (white arrows) in the neonate develops in almost precisely the same locations as does the T1 shortening.
B, T1-weighted image shows T1 shortening (black arrows) attributable to myelination.
An understanding of the development and structure of myelin is helpful in explaining the changing MR signals observed during brain development. The mature myelin sheath does not develop directly from the oligodendroglial cell membrane. The first myelin laid down by oligodendroglia represents a transitional membrane with properties that are intermediate between those of mature compact myelin and the oligodendroglial cell membrane. These transitional forms have less MBP, more MAG, and more 2,3-cyclic nucleotide 3-phosphodiesterase. The amount of PLP seems to stay the same, although some PLP in immature myelin is in the configuration of its isoform, DM20 (2) (the functional significance of having DM20 in place of PLP in immature myelin is unknown). As the maturation of myelin ensues, proteins mature earlier and lipids later (2). However, the maturation of the lipid bilayer seems to antedate the compaction of the myelin (2, 9).
At least two distinct populations of water molecules are known to contribute to MR images of white matter. The first is composed of water located within the myelin sheath; this has relatively short T1 and T2 relaxation times, probably because much of it forms transient hydrogen bonds with hydroxyl and ketone moieties of proteins and surface lipids. The second population of water molecules is composed of intraaxonal and interstitial water (ie, water outside of the myelin sheath); this second population likely contains a greater proportion of free water (not bound to macromolecules) and, thus, has longer T1 and T2 relaxation times (34). In the time scale of typical T1-weighted images, myelin water can diffuse across axonal and myelin membranes and interact with water molecules in the other compartments. It has been shown that the T1 shortening in developing white matter occurs at a time when the concentration of cholesterol and glycolipids is increasing in myelin (20, 35). Galactocerebrosides, which are among the most prevalent glycolipids in myelin, and cholesterol have several hydroxyl and ketone moieties per molecule to interact with free water molecules. In addition, galactocerebrosides (36) and cholesterol (37) have been shown to cause marked T1 shortening and magnetization transfer (36) in vitro. Thus, the interaction of water with the cholesterol, galactocerebrosides, and proteins of the myelin bilaminar membrane is most likely responsible for the early T1 shortening observed in developing white matter. In addition, these interactions are the likely cause of the increased magnetization transfer that is seen to accompany myelination in the developing brain (33). At the relatively long echo times used in acquiring T2-weighted images in neonates, the signal from protons of bound water molecules has largely dissipated and only the signal from axonal and extracellular water is likely to contribute to the MR signal (38). Furthermore, the increasing hypointensity seen on T2-weighted images of the maturing brain correlates temporally with chemical maturation of the myelin sheath, specifically with tightening of the myelin spiral around the axon (associated with conformational changes of myelin proteins) and saturation of some of the polyunsaturated fatty acids within the myelin membranes (20, 39–41). If this T2 signal is entirely from extracellular and axonal water, it may reflect decreasing proton density. This decrease in proton density is likely related to the further chemical maturation of myelin, to decreasing axonal water secondary to microtubule and microfilament production, or to decreasing extracellular free water secondary to myelin production (myelinated axons occupy considerably more space than unmyelinated axons) and the elaboration of glia and glial processes in the white matter.
Other factors are likely to be of importance, as well. Proper packing of lipids in the bilaminar membrane (Fig 3) is critical to the stability of myelin (8). Moreover, alteration of the packing of these lipids, by lengthening the hydrocarbon chains or adding additional double bonds to the chains, results in prolongation of T2 relaxation times (8), a point that is important in the imaging of patients with inborn errors of peroxisomal metabolism.
With increasing maturity of the brain, there is less motion of water molecules, resulting in a decrease in the ADC of brain water (30, 31, 42). In addition, the motion of the water molecules becomes increasingly anisotropic (30, 31, 42). The reduced ADC probably results from a number of factors. The size of the extracellular space is diminished because of the proliferation of myelin and the increased size of maturing neurons and glia. Another likely cause is a physical restriction of water motion by the multiple layers of the hydrophobic myelin membrane. This restriction of water motion by the myelin sheath is also postulated to be a major cause of the increasing anisotropy of water diffusion in the developing brain; water movement is restricted perpendicular to the myelin membranes but not parallel to them. However, it has been shown that diffusion anisotropy develops before any myelin can be detected by either histochemistry or electron microscopy (43). This observation has been attributed to two major factors. The first is the significant increase in the number of oligodendrocytes surrounding fascicles of axons immediately before myelination takes place; the oligodendrocytes themselves can restrict water motion perpendicular to the axons. The second is the development of intraaxonal macromolecules and functional ionic channels immediately before myelination; this combination of factors is postulated to make diffusion of water through the axon more difficult (43).
Disease
How do the factors herein discussed relate to the MR imaging appearance of myelin disease? I present a few examples of disorders in which the underlying biology is fairly well understood to help explain the correlation between imaging and myelin, as well as the complexity of these issues.
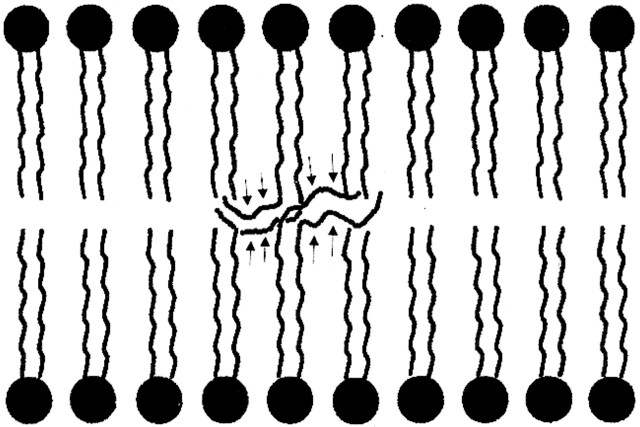
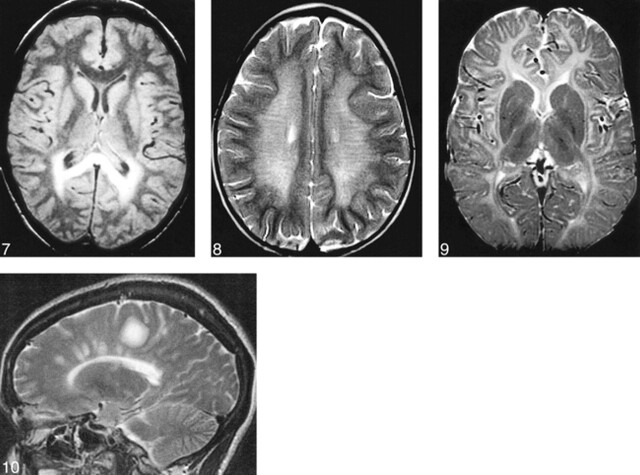
Adrenoleukodystrophy (ALD) is the most commonly occurring leukodystrophy in children, affecting approximately one in 20 000 boys (44). This X-linked disorder is caused by a mutation of the gene encoding a peroxisomal membrane protein. This protein (called ALD protein) is necessary for transferring very long chain fatty acids (24–26 carbon atoms in the chain) into peroxisomes, where they are normally metabolized into shorter chain fatty acids for purposes, among others, of incorporation into the bilaminar membrane of myelin. In the CNS, ALD protein is detected in all glial cells, including microglia and oligodendrocytes. However, its expression in oligodendrocytes is limited to the corpus callosum, internal capsule, and anterior commissure, locations that correlate well with the locations of demyelination in affected children (45). It is postulated that these very long chain fatty acids are incorporated into the bilaminar membrane of myelin in these locations, resulting in destabilization of the membrane (Fig 6) (46). Some event then triggers an inflammatory response by dysfunctional microglia (in which ALD protein is strongly expressed [45]) that seems to stimulate consequent demyelination. Ultimately, in most patients, the bilaminar membrane breaks down and demyelination ensues in those specific regions where the ALD gene is mutated in oligodendrocytes. MR imaging shows T2 prolongation during the early stages of the disease, usually beginning in the splenium of the corpus callosum and spreading into the occipital white matter (Fig 7). T2 prolongation is also commonly seen in the corticospinal and corticopontine tracts (47). The T2 prolongation may be due to myelin breakdown, inflammatory infiltrate, or both. The inflammatory component results in localized edema and some local mass effect. If contrast material is administered, a rim of enhancement is seen at the site of inflammation, at the junction of the normal brain and the region of T2 prolongation. The region of inflammation will show slightly reduced diffusion and will show essentially no change in magnetization transfer. As the disease and the demyelination progress, the mass effect disappears and is replaced by localized atrophy. In addition, the ADC increases, and both diffusion anisotropy and magnetization transfer are reduced.
fig 6.
Schematic of postulated membrane destabilization by very long chain fatty acids in ALD. As a result of the inability to import very long chain fatty acids into the peroxisome, they are not broken down into long chain fatty acids. When the very long chain fatty acids (arrows) are incorporated into the bilaminar membrane of myelin, the thermodynamic stability of the membrane is lessened, theoretically making myelin easier to break down.
fig 7.
ALD in an 8-year-old patient. Axial-view spin-echo (2500/30) image shows T2 prolongation in the callosal splenium and forceps major, regions where high concentrations of ALD protein are expressed.fig 8. MLD in a 2-year-old patient. Axial-view spin-echo (2500/80) image shows T2 prolongation, representing loss of myelin, in the central hemispheric white matter. The subcortical white matter is relatively spared. The radial stripes extending through the hemispheres are characteristic of the MR imaging appearance in this disorder.fig 9. Pelizaeus-Merzbacher disease in a 5-year-old patient. Axial-view spin-echo (600/15) image shows a paucity of myelin, with hyperintensity only in the internal capsules and optic radiations (compare with fig 4).fig 10. MS. Parasagittal fast spin-echo (3500/102) image shows differing degrees of hyperintensity in the large tumefactive plaque in the posterior frontal lobe, possibly representing different degrees of myelin and axonal destruction.
Metachromatic leukodystrophy (MLD) is an autosomal recessive disorder of myelin that is caused by deficient activity of the lysosomal enzyme arylsulfatase A. Patients may present at any age, depending on the level of activity of the enzyme (lower activity typically means earlier presentation). In the most common late infantile form, patients present with gait abnormalities, ataxia, nystagmus, and hypotonia. Eventually, they develop diffuse spasticity, with pathologic reflexes (48). Myelin is usually formed normally in MLD. However, the reduced activity of arylsulfatase A, the enzyme that catalyzes the sulfatide to cerebroside, which can be further degraded, results in accumulation of sulfatides within the oligodendrocyte. Although sulfatide is a normal oligodendrocyte membrane lipid, high levels of sulfatide result in an excess of the chemical within the cell membrane and a reduced amount of galactocerebroside (normally formed by the breakdown of sulfatide). This progressive increase of sulfatide and decrease of cerebroside results in increasing instability of the myelin membrane with ultimate demyelination. Another factor that may contribute to the demyelination is oligodendroglial cell injury of death resulting from the progressive accumulation of sulfatides within oligodendroglial lysosomes. Ultimately, the lysosomes degenerate and release their highly toxic enzymes into the cytoplasm, initiating cascades of molecular reactions that lead to cell death (49, 50). Pathologic examination of affected brains shows demyelination, predominantly within the centrum semiovale. The demyelination is most severe in the periventricular white matter, diminishing in regions closer to the cortex. The subcortical U fibers tend to be spared early in the course of the disease. Histologic examination shows complete or nearly complete loss of myelin in the most severely affected areas, with relative sparing of the axons. A prominent astrogliosis is present. Oligodendroglia are absent from affected regions (50). Imaging reflects the biochemical and pathologic changes, showing radially oriented stripes of T2 prolongation (Fig 8). It is uncertain whether the stripes of more normal-appearing (hypointense) tissue represent areas of relative myelin sparing; these are, however, characteristic of the disorder.
Pelizaeus-Merzbacher disease is a rare, X-linked disease characterized by dysmyelination of the CNS that is associated with mutations in the PLP gene, located at Xq22. The disease can be caused by either deletion of the gene, mutations (missense, frameshift, insertion, deletion, or nonsense) of the gene, or duplication of the gene (the most common mutation, resulting in increased dosage of the PLP protein) (51). When the gene is completely deleted, affected children have a relatively mild form of disease, despite the hypomyelination (52, 53). In mouse models of PLP deletions, myelin is formed (albeit with abnormal intraperiod lines) but axonal swelling and degeneration develop during the second month of life (51). This implies that PLP has a metabolic, as well as structural, function. Patients with mutations and duplications of the PLP gene have variable clinical phenotypes and variable hypomyelination, depending on the location and type of mutation (51). In general, more severe abnormalities of protein folding or changes in the protein at locations of key chemical interactions result in more severe phenotypes. Most symptomatic children have abnormalities in the large protein loop that is in the extracellular space and interacts with the adjacent myelin spiral (51). In addition to the obvious effects that such changes will have on the physical structure of the myelin, it has been suggested that misfolded proteins are less easily transported to the cell membrane from the endoplasmic reticulum of the oligodendroglial cells (54). The accumulation of the misfolded proteins in the cell may trigger oligodendroglial apoptosis and consequent demyelination (55). From an MR imaging perspective, the reduction of normal myelin and normal oligodendroglial cells in the brain results in delayed myelination (Fig 9). In contrast to ALD, there is no inflammatory infiltrate, so less free water is present and the cerebral and cerebellar white matter do not appear as bright and have less prolongation of T1 and T2. As a result of the marked reduction of myelin, the ADC is increased and both diffusion anisotropy and magnetization transfer are decreased compared with age-matched control subjects. It is of interest to note that glial cell progenitors have been introduced into the lateral ventricles of a rat model of Pelizaeus-Merzbacher disease, resulting in increased myelination in widespread sites throughout the brain (56). If this technique proves adaptable to human therapy, neuroradiologists may be called on to monitor response by using quantitative techniques such as magnetization transfer imaging.
No known human disease results from abnormality of MBP. An animal model, the shiverer mouse, has an autosomal recessive mutation of the MPB gene, which, in homozygous conditions, results in a nearly total lack of myelin in the CNS. Affected mice have generalized tremors beginning at age 2 weeks and subsequently develop seizures (9). Histopathologic examination reveals markedly reduced CNS myelin. The residual myelin is found to be tightly compacted at the intraperiod line but shows vacuolation at the normal site of the major dense line (57). A human disorder, the 18q- syndrome, is the result of hemizygous deletion of the long arm of chromosome 18, the site of the MBP gene. Several studies have shown abnormal T2 prolongation of white matter in patients with this disorder (58, 59) and have postulated that reduced myelin secondary to the absence of one copy of the MBP gene may be responsible for the MR imaging findings. However, no pathologic correlations have confirmed this speculation (60).
No discussion of myelin and its disorders would be complete without a brief discussion of multiple sclerosis (MS). MS is the most common demyelinating disease of the CNS. In patients with this disorder, normal myelin is formed but subsequently broken down by presumably autoimmune mechanisms (61). The underlying pathogenesis of MS is complex and incompletely understood. The disorder probably results from a combination of genetic and environmental factors. An association has been found between MS and certain human leukocyte antigen alleles, which have been implicated in a number of human immunologic disorders. In addition, the early phases of MS exacerbations are characterized by the invasion of the brain by inflammatory cells, thought to be caused by abnormalities of immune regulation (61). Epidemiologic studies have shown that environmental factors are also important in association with MS (62). It is postulated that exposure to viral antigens, particularly in persons younger than 15 years, generates antibodies that may subsequently be reactivated to attack similar antigens in myelin (50). The precise component of myelin that is attacked in cases of MS is not known, but it is likely that different viral antigens may cross-react with many different components. Antibodies reacting with myelin proteins (in particular myelin oligodendrocyte glycoprotein and MBP), oligodendroglia, and glycolipids have been observed in the CSF of patients with MS (63–66), but no single MS-specific antigen has been identified (50). Whatever the underlying cause, it is clear that one of the earliest manifestations is an infiltration of lymphocytes from venules into the surrounding brain tissue. Subsequently, other inflammatory cells infiltrate the tissue and generate edema, followed by demyelination, and reactive astrogliosis. After the initial event, the myelin may remain completely normal. Eventually, in some sites, the myelin molecule breaks down and the myelin sheath undergoes vacuolization, with separation of the adjacent spirals (64). Ultimately, the axon degenerates as well, because of the symbiotic relationship of the myelin and the axon (64).
The pathologic manifestations are reflected in imaging studies. Early inflammatory disease shows local interstitial edema (T2 prolongation) (Fig 10), often with blood-brain–barrier breakdown (enhancement) due to local inflammation (67), diffusion anisotropy is reduced (68), and proton spectroscopy shows increased choline and lactate (69). If myelin is still intact at this time, magnetization transfer ratios may be normal or only mildly reduced (70, 71). As the myelin breaks down and the axons degenerate, sharply demarcated foci of T1 prolongation develop (72), differences in T2 relaxation develop (Fig 10), magnetization transfer ratios are further reduced (70), apparent diffusion is increased (68), and N-acetylaspartate is reduced as revealed by MR spectroscopy (69). T2 prolongation shows no correlation with axonal loss (73); however, T1 prolongation and reduced magnetization transfer both show strong correlation with axonal loss (73). Correlation has been also shown between reduction in magnetization transfer ratio and clinical disability scores (74, 75). It is interesting to note that even normal-appearing white matter can have reduced magnetization transfer in patients with MS (76). Although it has been suggested that this is the result of inherently abnormal myelin in patients with MS, the normal chemical composition of their myelin indicates that the reduced magnetization transfer is more likely the result of widespread abnormalities that are too small to be detected by standard MR imaging (50).
Summary
Abnormalities of myelin can cause a wide variety of disorders of the nervous system. MR imaging is a powerful tool for the study of myelin and its disorders. However, only by understanding the physiologic properties and structure of myelin can we use MR imaging to its fullest capacity for studying patients with myelin disorders. In this review, I have discussed the structure of myelin as it relates to MR imaging of normal myelination and to neurologic disorders resulting from abnormalities of myelin. Thinking of myelin and its disorders in this manner will be critical to using MR imaging techniques optimally to diagnose and study these disorders further.
Footnotes
Address reprint requests to A. James Barkovich, MD, Neuroradiology, Box 0628, University of Southern California, San Francisco, 505 Parnassus Avenue, San Francisco, CA 94143–0628.
References
- 1.Brant-Zawadzki M, Enzmann DR. Using computed tomography of the brain to correlate low white matter attenuation with early gestational age in neonates. Radiology 1981;139:105-108 [DOI] [PubMed] [Google Scholar]
- 2.Morell P, Quarles RH, Norton WT. Myelin formation, structure, and biochemistry. In: Siegel GJ, ed. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. 5th ed. New York: Raven Press 1994 117-143
- 3.Brady ST, Witt AS, Kirkpatrick LL, et al. Formation of compact myelin is required for maturation of the axonal cytoskeleton. J Neurosci 1999;19:7278-7288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirchner DA, Blaurock AE. Organization, phylogenetic variations and dynamic transitions of myelin. In: Martenson RE, ed. Myelin: Biology and Chemistry. Boca Raton: CRC 1991 413-448
- 5.Kirschner DA, Ganser AL. Myelin labeled with mercuric chloride: asymmetric localization of phosphatidylethanolamine plasmalogen. J Mol Biol 1982;157:635-658 [DOI] [PubMed] [Google Scholar]
- 6.Braun PE. Molecular organization of myelin. In: Morell P, ed. Myelin. New York: Plenum Press 1984 97-116
- 7.van der Knaap MJ, Valk J. Myelin and white matter. In: van der Knaap MJ, Valk J, eds. Magnetic Resonance of Myelin, Myelination, and Myelin Disorders. 2nd ed. Berlin: Springer 1995 1-17
- 8.Lee J, Barry JA. Influence of membrane lipid packing on T2-weighted magnetic resonance images: study of relaxation parameters in model membrane systems. Magn Reson Med 1996;36:420-426 [DOI] [PubMed] [Google Scholar]
- 9.Jacobson M. Developmental Neurobiology. 3rd ed. New York: Plenum Press 1991 776
- 10.Weimbs T, Stoffel W. Proteolipid protein (PLP) of CNS myelin: positions of free, disulfide bonded, and fatty acid thioester-linked cysteine residues: implications for the membrane topology of PLP. Biochemistry 1992;31:12289-12296 [DOI] [PubMed] [Google Scholar]
- 11.Knapp PE. Proteolipid protein: is it more than just a structural component of myelin? Dev Neurosci 1996;18:297-308 [DOI] [PubMed] [Google Scholar]
- 12.Griffiths I, Klugmann M, Anderson T, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 1998;280:1610-1613 [DOI] [PubMed] [Google Scholar]
- 13.Coetzee T, Suzuki K, Popko B. New perspectives on the function of myelin galactolipids. Trends Neurosci 1998;21:126-130 [DOI] [PubMed] [Google Scholar]
- 14.Quarles RH. Glycoproteins of myelin sheaths. J Mol Neurosci 1997;8:1-12 [DOI] [PubMed] [Google Scholar]
- 15.Li C, Tropak MB, Gerlai R, et al. Myelination in the absence of myelin-associated glycoprotein. Nature 1994;369:747-750 [DOI] [PubMed] [Google Scholar]
- 16.Schnaar RL, Collins BE, Wright LP, et al. Myelin-associated glycoprotein binding to gangliosides: structural specificity and functional implications. Ann N Y Acad Sci 1998;845:92-105 [DOI] [PubMed] [Google Scholar]
- 17.Quarles RH. Myelin-associated glycoprotein in development and disease. Dev Neurosci 1985;6:285-303 [DOI] [PubMed] [Google Scholar]
- 18.Trapp BD. Distribution of the myelin-associated glycoprotein and P0 protein during myelin compaction in quaking mouse peripheral nerve. J Cell Biol 1988;107:675-685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konat GW. Chromatin structure and transcriptional activity of MAG gene. Acta Neurobiol Exp (Warsz) 1996;56:281-285 [DOI] [PubMed] [Google Scholar]
- 20.Barkovich AJ, Kjos BO, Jackson DE Jr, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology 1988;166:173-180 [DOI] [PubMed] [Google Scholar]
- 21.Bird CR, Hedberg M, Drayer BP, Keller PJ, Flom RA, Hodak JA. MR assessment of myelination in infants and children: usefulness of marker sites. AJNR Am J Neuroradiol 1989;10:731-740 [PMC free article] [PubMed] [Google Scholar]
- 22.Christophe C, Muller MF, Baleriaux D, et al. Mapping of normal brain maturation in infants on phase-sensitive inversion-recovery images. Neuroradiology 1990;32:173. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich RB, Bradley WG, Zagaroza EJ, et al. MR evaluation of early myelination patterns in normal and developmentally delayed infants. AJR Am J Roentgenol 1988;150:889-896 [DOI] [PubMed] [Google Scholar]
- 24.Holland BA, Haas DK, Norman D, Brant-Zawadzki M, Newton TH. MRI of normal brain maturation. AJNR Am J Neuroradiol 1986;7:201-208 [PMC free article] [PubMed] [Google Scholar]
- 25.McArdle CB, Richardson CJ, Nicholas DA, Mirfakhraee M, Hayden CK, Amparo EG. Developmental features of the neonatal brain: MR imaging: part I: gray-white matter differentiation and myelination. Radiology 1987;162:223-229 [DOI] [PubMed] [Google Scholar]
- 26.van der Knaap MS, Valk J. MR imaging of the various stages of normal myelination during the first year of life. Neuroradiology 1990;31:459-470 [DOI] [PubMed] [Google Scholar]
- 27.Martin E, Kikinis R, Zuerrer M, et al. Developmental stages of the human brain: an MR study. J Comput Assist Tomogr 1988;12:917-922 [DOI] [PubMed] [Google Scholar]
- 28.Martin E, Krassnitzer S, Kaelin P, Boesch C. MR imaging of the brainstem: normal postnatal development. Neuroradiology 1991;33:391-395 [DOI] [PubMed] [Google Scholar]
- 29.Barkovich AJ. MR of the normal neonatal brain: assessment of deep structures. AJNR Am J Neuroradiol 1998;19:1397-1403 [PMC free article] [PubMed] [Google Scholar]
- 30.Nomura Y, Sakuma H, Takeda K, Tagami T, Okuda Y, Nakagawa T. Diffusional anisotropy of the human brain assessed with diffusion-weighted MR: relation with normal brain development and aging. AJNR Am J Neuroradiol 1994;15:231-238 [PMC free article] [PubMed] [Google Scholar]
- 31.Sakuma H, Nomura Y, Takeda K, et al. Adult and neonatal human brain: diffusional anisotropy and myelination with diffusion-weighted MR imaging. Radiology 1991;180:229-233 [DOI] [PubMed] [Google Scholar]
- 32.Neil JJ, Shiran SI, McKinstry RC, et al. Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology 1998;209:57-66 [DOI] [PubMed] [Google Scholar]
- 33.Chew WM, Rowley HA, Barkovich AJ. Magnetization transfer contrast imaging in pediatric patients. Radiology 1992;185:2811523326 [Google Scholar]
- 34.Whittall KP, MacKay AL, Graeb DA, Nugent RA, Li DK, Paty DW. In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med 1997;37:34-43 [DOI] [PubMed] [Google Scholar]
- 35.Poduslo SE, Jang Y. Myelin development in infant brain. Biochem Res 1984;9:1615-1626 [DOI] [PubMed] [Google Scholar]
- 36.Kucharczyk W, Macdonald P, Stanisz G, Henkelman RM. Relaxivity and magnetization transfer of white matter lipids at MR imaging: importance of cerebrosides and pH. Radiology 1994;192:521-529 [DOI] [PubMed] [Google Scholar]
- 37.Koenig SH, Brown RD III, Spiller M, Lundbom N. Relaxometry of the brain: why white matter appears bright on MRI. Magn Reson Med 1990;14:482-495 [DOI] [PubMed] [Google Scholar]
- 38.Baratti C, Barnett AS, Pierpaoli C. Comparative MR imaging study of brain maturation in kittens with T1, T2, and the trace of the diffusion tensor. Radiology 1999;210:133-142 [DOI] [PubMed] [Google Scholar]
- 39.Husted C, Montez B, Le C, Moscarello MA, Oldfield E. Carbon-13 “magic-angle” sample-spinning nuclear magnetic resonance studies of human myelin, and model membrane systems. Magn Reson Med 1993;29:168-178 [DOI] [PubMed] [Google Scholar]
- 40.Husted C. Carbon-13 Magic Angle Spinning NMR Studies of Myelin Membranes. [Phd thesis]. Urbana: University of Illinois at Urbana-Champaign 1991
- 41.Barkovich AJ. Brain development: normal and abnormal. In: Atlas SW, ed. Magnetic Resonance Imaging of the Brain and Spine. New York: Raven Press 1991 129-175
- 42.Wimberger D, Roberts TP, Kucharczyk J, Barkovich AJ, Kozniewska E, Prayer LM. Diffusion-weighted imaging at 4.7 T in the assessment of brain maturation in albino rats. Presented at the Society of Magnetic Resonance Imaging meeting, San Francisco, 1993
- 43.Wimberger DM, Roberts TP, Barkovich AJ, Prayer LM, Moseley ME, Kucharczyk J. Identification of “premyelination” by diffusion-weighted MRI. J Comput Assist Tomogr 1995;19:28-33 [DOI] [PubMed] [Google Scholar]
- 44.Dubois-Dalcq M, Feigenbaum V, Aubourg P. The neurobiology of X-linked adrenoleukodystrophy, a demyelinating peroxisomal disorder. Trends Neurosci 1999;22:4-12 [DOI] [PubMed] [Google Scholar]
- 45.Fouquet F, Zhou JM, Ralston E, et al. Expression of the adrenoleukodystrophy protein in the human and mouse central nervous system. Neurobiol Dis 1997;3:271-285 [DOI] [PubMed] [Google Scholar]
- 46.Ho JK, Moser H, Kishimoto Y, Hamilton JA. Interactions of a very long chain fatty acid with model membranes and serum albumin: implications for the pathogenesis of adrenoleukodystrophy. J Clin Invest 1995;96:1455-1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar AJ, Rosenbaum AE, Naidu S, et al. Adrenoleukodystrophy: correlating MR imaging with CT. Radiology 1987;165:497-504 [DOI] [PubMed] [Google Scholar]
- 48.Barkovich AJ. Toxic and metabolic brain disorders. In: Barkovich AJ, ed. Pediatric Neuroimaging. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2000
- 49.Gieselmann V, Polten A, Kreysing J, von Figura K. Molecular genetics of metachromatic leukodystrophy. J Inherit Metab Dis 1994;17:500-509 [DOI] [PubMed] [Google Scholar]
- 50.van der Knaap MS, Valk J. Magnetic Resonance of Myelin, Myelination, and Myelin Disorders. 2nd ed. Berlin: Springer 1995;
- 51.Woodward K, Malcolm S. Proteolipid protein gene: Pelizaeus-Merzbacher disease in humans and neurodegeneration in mice. Trends Genet 1999;15:125-129 [DOI] [PubMed] [Google Scholar]
- 52.Sistermans EA, de Wijs IJ, de Coo RF, Smit LM, Menko FH, van Oost BA. A (G-to-A) mutation in the initiation codon of the proteolipid protein gene causing a relatively mild form of Pelizaeus-Merzbacher disease in a Dutch family. Hum Genet 1996;97:337-339 [DOI] [PubMed] [Google Scholar]
- 53.Raskind WH, Williams CA, Hudson LD, Bird TD. Complete deletion of the proteolipid protein gene (PLP) in a family with X-linked Pelizaeus-Merzbacher disease. Am J Hum Genet 1991;49:1355-1360 [PMC free article] [PubMed] [Google Scholar]
- 54.Gow A, Lazzarini RA. A cellular mechanism governing the severity of Pelizaeus-Merzbacher disease. Nat Genet 1996;13:422-428 [DOI] [PubMed] [Google Scholar]
- 55.Gow A, Southwood CM, Lazzarini RA. Disrupted proteolipid protein trafficking results in oligodendrocyte apoptosis in an animal model of Pelizaeus-Merzbacher disease. J Cell Biol 1998;140:925-934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Learish R, Brüstle O, Zhang S-C, Duncan I. Intraventricular transplantation of oligodendrocyte progenitors into a fetal myelin mutant results in widespread formation of myelin. Ann Neurol 1999;46:716-722 [PubMed] [Google Scholar]
- 57.Roth HJ, Hunkeler MJ, Campagnoni AT. Expression of myelin basic protein genes in several dysmyelinating mouse mutants during early postnatal brain development. J Neurochem 1985;45:572-580 [DOI] [PubMed] [Google Scholar]
- 58.Loevner LA, Shapiro RM, Grossman RI, Overhauser J, Kamholz J. White matter changes associated with deletions of the long arm of chromosome 18 (18q- syndrome): a dysmyelinating disorder? AJNR Am J Neuroradiol 1996;17:1843-1848 [PMC free article] [PubMed] [Google Scholar]
- 59.Gay CT, Hardies LJ, Rauch RA, et al. MRI demonstrates incomplete myelination in 18q- syndrome: evidence for myelin basic protein haploinsuffiency. Am J Med Genet 1997;74:422-431 [DOI] [PubMed] [Google Scholar]
- 60.Becker LE. MR findings in the central nervous system of patients with a deletion of the long arm of chromosome 18(18q-) [letter]. AJNR Am J Neuroradiol 1998;19:399. [PMC free article] [PubMed] [Google Scholar]
- 61.Chiappa KH, Parker SW, Shahani BT. Pathoneurophysiology of multiple sclerosis. In: Koetsier JC, ed. Handbook of Clinical Neurology. Amsterdam: Elsevier 1985 131-145
- 62.Kurtzke JF. Epidemiology of multiple sclerosis. In: Koetsier JC, ed. Handbook of Clinical Neurology. Amsterdam: Elsevier 1985 259-287
- 63.Reindl M, Linington C, brehm U, et al. Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain 1999;122:2047-2056 [DOI] [PubMed] [Google Scholar]
- 64.Raine CS, Canella B, Hauser SL, Genain CP. Demyelination in primate autoimmune encephalomyelitis and acute multiple sclerosis lesions: a case for antigen-specific antibody mediation. Ann Neurol 1999;46:144-160 [DOI] [PubMed] [Google Scholar]
- 65.Lucchinetti CF, Bruck W, Rodriguez M, Lassmann H. Distinct patterns of multiple sclerosis pathology indicates heterogeneity of pathogenesis. Brain Pathol 1996;6:259-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Genain CP, Canella B, Hauser S, Raine C. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med 1999;5:153-154 [DOI] [PubMed] [Google Scholar]
- 67.Grossman RI, Braffman BH, Brorson JR, Goldberg HI, Silverberg DH, Gonzalez-Scarano F. Multiple sclerosis: serial study of gadolinium-enhanced MR imaging. Radiology 1988;169:117-122 [DOI] [PubMed] [Google Scholar]
- 68.Werring DJ, Clark CA, Barker gJ, Thompson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology 1999;52:1626-1632 [DOI] [PubMed] [Google Scholar]
- 69.Bitsch A, Bruhn H, Vougioukas V, et al. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol 1999;20:1619-1627 [PMC free article] [PubMed] [Google Scholar]
- 70.Dousset V, Grossman RI, Ramer KN, et al. Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology 1992;182:483-491 [DOI] [PubMed] [Google Scholar]
- 71.Filippi M, Rocca MA, Rizzo G, et al. Magnetization transfer ratios in multiple sclerosis lesions enhancing after different doses of gadolinium. Neurology 1998;50:1289-1293 [DOI] [PubMed] [Google Scholar]
- 72.van Walderveen MAA, Kamphorst W, Scheltens P, et al. Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology 1998;50:1282-1288 [DOI] [PubMed] [Google Scholar]
- 73.van Waesberghe J, Kamphorst W, De Groot C, et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol 1999;46:747-754 [DOI] [PubMed] [Google Scholar]
- 74.van Buchem MA, Grossman RI, Armstrong C, et al. Correlation of volumetric magnetization transfer imaging with clinical data in MS. Neurology 1998;50:1609-1617 [DOI] [PubMed] [Google Scholar]
- 75.Gass A, Barker GJ, Kidd D, et al. Correlation of magnetization transfer ratio with clinical disability in multiple sclerosis. Ann Neurol 1994;36:62-67 [DOI] [PubMed] [Google Scholar]
- 76.Loevner LA, Grossman RI, Cohen JA, Lexa FJ, Kessler D, Kolson DL. Microscopic disease in normal-appearing white matter on conventional MR images in patients with multiple sclerosis: assessment with magnetization-transfer measurements. Radiology 1995;196:511-515 [DOI] [PubMed] [Google Scholar]