Abstract
BACKGROUND AND PURPOSE: Abuse of the popular recreational drug “Ecstasy” (MDMA) has been linked to the occurrence of cerebrovascular accidents. It is known that MDMA alters brain serotonin (5-HT) concentrations and that brain postsynaptic 5-HT2 receptors play a role in the regulation of brain microvasculature. Therefore, we used brain imaging to find out whether MDMA use predisposes one to cerebrovascular accidents by altering brain 5-HT neurotransmission.
METHODS: The effects of MDMA use on brain cortical 5-HT2A receptor densities were studied using [123I]R91150 single-photon emission CT in 10 abstinent recent MDMA users, five former MDMA users, and 10 healthy control subjects. Furthermore, to examine whether changes in brain 5-HT2A receptor densities are associated with alterations in blood vessel volumes, we calculated relative cerebral blood volume maps from dynamic MR imaging sets in five MDMA users and six healthy control subjects.
RESULTS: An analysis of variance revealed that mean cortical [123I]R91150 binding ratios were significantly lower in recent MDMA users than in former MDMA users and control subjects. This finding suggests down-regulation of 5-HT2 receptors caused by MDMA-induced 5-HT release. Furthermore, in MDMA users, low cortical 5-HT2 receptor densities were significantly associated with low cerebral blood vessel volumes (implicating vasoconstriction) and high cortical 5-HT2 receptor densities with high cerebral blood vessel volumes (implicating vasodilatation) in specific brain regions.
CONCLUSION: These findings suggest a relationship between the serotonergic system and an altered regulation of 5-HT2 receptors in human MDMA users. MDMA users may therefore be at risk for cerebrovascular accidents resulting from alterations in the 5-HT neurotransmission system.
Recently, several case reports have linked the abuse of the popular recreational party drug 3,4-methylenedioxymethamphetamine (MDMA, or “Ecstasy”) to the occurrence of cerebrovascular accidents (1–8). The brain area most vulnerable to the vascular effects of MDMA is the globus pallidus, a region rich in serotonin (5-HT) nerve terminals (9, 10). Considerable evidence has accumulated over the years strongly pointing to the involvement of 5-HT and 5-HT2 receptors in the regulation of brain microcirculation (11–12). MDMA induces release of 5-HT from serotonergic neurons. However, abuse of this drug eventually leads to loss of serotonergic neurons, causing 5-HT depletion and a compensatory up-regulation of postsynaptic 5-HT2 receptors. It has therefore been suggested in several reports that MDMA abuse may predispose to cerebrovascular disease as a result of MDMA-induced effects on brain 5-HT concentrations and 5-HT2 receptors (2, 9, 10, 13). Advances in neuroimaging techniques, such as single-photon emission CT (SPECT), have made it possible to study 5-HT2 receptors in the living human brain, using iodine-123 labeled R91150. [123I]R91150 binds selectively and with high affinity to the 5-HT2A receptor subtype. Cortical binding of [123I]-5-I-R91150 for 5-HT2A receptors is specific and reversible, as shown by inhibition of binding by ritanserin and displacement by ketanserin (14). Moreover, by using cerebral blood volume (CBV) maps calculated from dynamic MR imaging sets, it is now possible to study relative CBV (rCBV) in the brain (15, 16), in which regional vasospasm will decrease rCBV values and vasodilatation will increase CBV values (17).
The aim of the present preliminary study was to use [123I]R91150 SPECT to investigate the effects of MDMA use on brain 5-HT2A receptor density, and, with the use of MR imaging sets, to ascertain whether these effects are associated with alterations in rCBV in abstinent recent MDMA users, former MDMA users, and healthy control subjects.
Methods
Participants
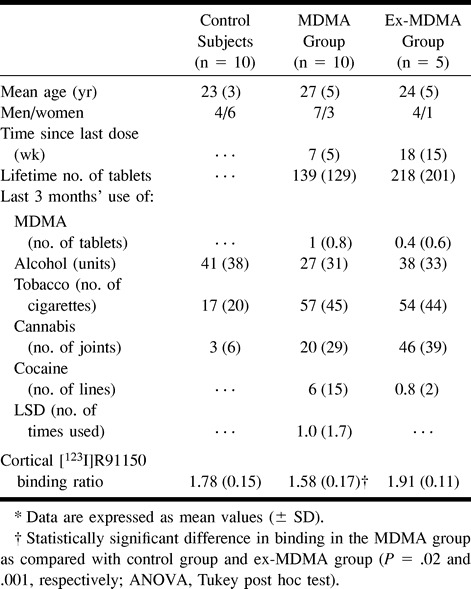
Fifteen participants who reported previous heavy use of MDMA (mean age, 26 years) and 10 age-matched control subjects (mean age, 23 years) (Table 1) were enrolled in the SPECT study. The eligibility criterion for the MDMA group was previous use of at least 50 tablets of MDMA. Ten participants had recently used this drug (MDMA group) and five former MDMA users had abstained from using MDMA (ex-MDMA group; Table 1). The eligibility criterion for the MDMA group was a drug-free interval of 1 week to 2 months prior to the study. Because animal studies have shown that down-regulation of 5-HT2 receptors persists for at least 1 month after the last intake of MDMA (18), the cut-off point of the drug-free interval for the ex-MDMA group was established at 2 months. The control group consisted of healthy subjects with no self-reported prior use of psychoactive drugs, including MDMA. Recruitment was through advertisements in local newspapers. Participants agreed to abstain from use of psychoactive drugs for at least 1 week before the study, and were asked to undergo urine drug screening (with an enzyme-multiplied immunoassay for amphetamines, barbiturates, benzodiazepine metabolites, cocaine and metabolites, opiates, and marijuana) before enrollment. After the testing of urine samples, exclusion criteria were a positive drug screen, pregnancy, severe medical or neuropsychiatric illness that precluded informed consent, claustrophobia, a cardiac pacemaker or surgical clip, and neuropsychiatric disease in which 5-HT has been implicated. All participants gave written informed consent.
TABLE 1:
5-HT2 receptor imaging: characteristics of participants and mean cortical [123I]R91150 binding ratios*
5-HT2 Receptor Imaging
For SPECT studies, the Strichman Medical Equipment 810X tomographic system was used (Strichman Medical Equipment, Inc., Medfield, MA). The transaxial resolution of this camera is 7.6 mm full-width at half-maximum of a line source in air, and the axial resolution is 13.5 mm. Each acquisition consisted of 15 slices acquired in a 128 × 128 matrix with a slice distance of 5 mm and a scanning time of 3 minutes per slice. The energy window was set at 135 to 190 keV. Subjects lay in the supine position with the head aligned parallel to the orbitomeatal line, and were positioned such that the scanning volume initially included the cerebellum. Acquisition of images began 2 hours after intravenous injection of approximately 140 MBq [123I]R91150 (radiolabeling as described by Busatto and coworkers [14]), a time at which specific binding is maximal and stable for up to 8 hours after injection. For assessment of the scans, reviewers were blinded to subject status. For analysis of [123I]R91150 binding, a standard template with regions of interest (ROIs) was constructed manually from coregistered MR images. For positioning, we used these MR images as a guide. Coregistration of MR images and SPECT scans was performed using the Hermes Multi Modality software package (Nuclear Diagnostics, Stockholm, Sweden). The template, including ROIs for the frontal, parietal, and occipital cortices, was placed on the three highest consecutive SPECT slices. An additional template was constructed with an ROI for the cerebellum. Mean signal density of the left and right cortices (mean counts per pixel of frontal, parietal, and occipital cortices) and of the cerebellum was determined. ROI analysis was performed by an investigator unaware of the participants' history. The uptake in the cerebellum, presumed free from 5-HT2A receptors, was used as a reference for background radioactivity (nonspecific binding plus free ligand). ROI/cerebellum activity ratios were calculated as a relative measure of specific binding to 5-HT2A receptors for a given brain region (14, 19).
Calculating rCBV Values
rCBV maps were calculated from dynamic MR imaging sets acquired with echo-planar spin-echo imaging after intravenous injection of gadolinium-based contrast material. MR images were obtained at 1.5 T. MR imaging was performed, on average, 6 hours before SPECT studies were obtained. An 18-gauge catheter was inserted into a large peripheral vein before MR imaging was performed. A saline drip was used to maintain the vein's patency. Gadopentetate dimeglumine (0.2 mmol/kg) was power-injected at a rate of 5 mL/s through the angiocatheter. A series of images (40 series of 12 slices in 64 seconds) was obtained at intervals of 1202 milliseconds using a lipid-suppressed spin-echo echo-planar pulse sequence (TR/TE = 800/54) before, during, and after injection of the contrast agent. Lipid suppression was used to suppress subcutaneous fat. We used a 128 × 128 × 12 matrix with a voxel size of 1.8 × 1.8 × 6.0 mm. After data collection, rCBV maps were derived on a voxel-by-voxel basis from the dynamic imaging sets (using software developed at MGH-NMR Center, Charlestown, MA) (20, 21). Because the susceptibility contrast rCBV mapping method yields relative rather than absolute values of rCBV, comparison among subjects is facilitated by reference to an internal standard. Analogous to previous studies (22, 23), normal white matter was used as this reference. To calculate rCBV/white matter, the ROIs of the various brain regions (left and right frontal and occipital cortices, white matter, putamen, and globus pallidus) were defined on rCBV maps by a radiologist unaware of the participants' history. Ratios were calculated by dividing the mean rCBV of the brain region by that of unilateral mean white matter. Because rCBV maps enable quantification of vascularization in relative terms (22), a high rCBV ratio implies high rCBV, or vasodilatation, whereas a low rCBV ratio implies vasoconstriction (17).
Statistics
Differences in mean cortical [123I]R91150 radioligand binding among groups were tested by one-way analysis of variance. Differences in rCBV values among groups were analyzed using an unpaired Student's t-test. The relationship between mean cortical [123I]R91150 radioligand binding and rCBV values in specific brain regions was investigated with Spearman's rank correlation, since it has the advantage that it does not specifically assess a linear association but a more general association. A P value less than .05 was taken to be significant with a two-tailed test. We analyzed all data with SPSS version 9.0 software (Statistical Package for the Social Sciences, Chicago, IL).
Results
5-HT2 Receptor Imaging
Participants in the MDMA and ex-MDMA group had used, on average, 139 ± 129 tablets and 218 ± 201 tablets of MDMA, respectively (Table 1). Participants in the MDMA and ex-MDMA had not used MDMA, on average, for 7 ± 5 weeks and 18 ± 15 weeks, respectively, before this investigation. All participants were right-handed.
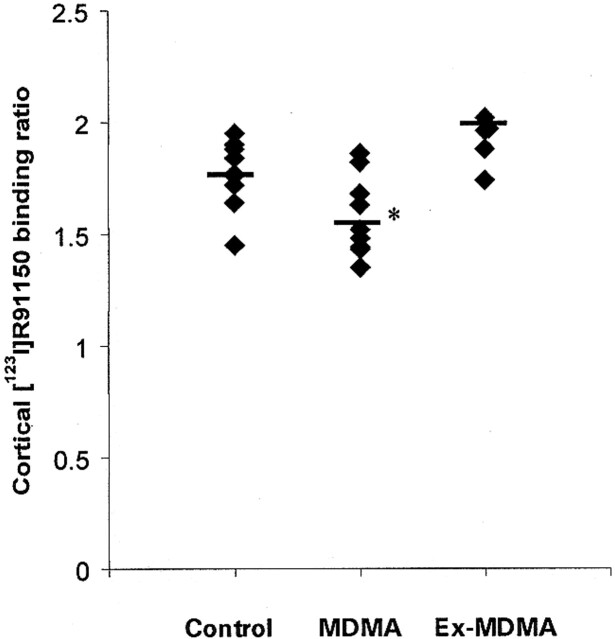
Left and right cortical [123I]R91150 binding did not differ significantly between control subjects and MDMA users. Therefore, mean cortical 5-HT2A receptor-binding ratios were calculated (average of left and right frontal, parietal, and occipital [123I]R91150 binding). Mean cortical 5-HT2A receptor binding ratios in the MDMA group were significantly lower than those in the ex-MDMA and control groups (P = .001, and P = .02, respectively). Mean cortical 5-HT2A receptor binding ratios were higher in the ex-MDMA group than in the control group, although this difference was not statistically significant (Table 1, Figs 1 and 2).
fig 1.
The mean and individual [123I]R91150 binding ratios in the cortex: control subjects versus MDMA and ex-MDMA users. Cortical binding ratios were calculated as cortical binding / binding in the cerebellum. Asterisk indicates a statistically significant difference in binding ratio as compared with control subjects and ex-MDMA users
rCBV Values
In addition to the SPECT studies, we performed dynamic MR imaging in a random sample of participants of the SPECT study to calculate rCBV values. Eventually, a random sample of five MDMA users (three recent users and two ex-MDMA users) and six healthy control subjects were enrolled in the MR imaging study (Table 2). No significant difference in left and right rCBV values was found between the control subjects and the MDMA users. Therefore, a mean of left and right cerebral rCBV values was calculated for the brain regions studied. Mean rCBV values for the MDMA users did not differ significantly from those obtained in control subjects in the brain regions studied (Table 2). The subgroup of two ex-MDMA users had higher rCBV values in the brain regions studied than did the recent MDMA users. Compared with the control subjects, the ex-MDMA users also had higher rCBV values in some specific brain regions (Fig 3).
TABLE 2:
rCBV values: characteristics of participants and mean rCBV ratio in brain areas studied*
fig 3.
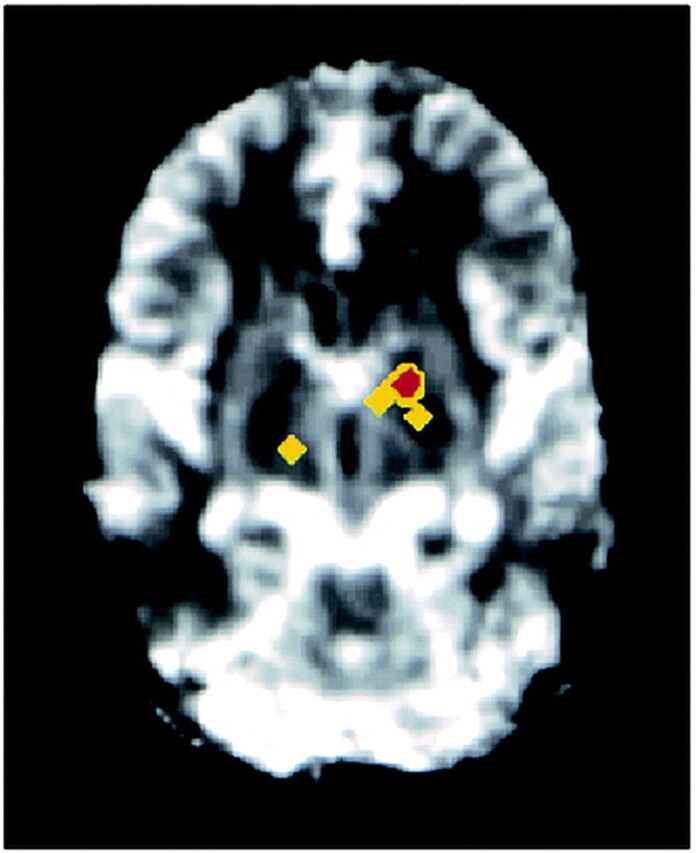
Higher rCBV values are observed in the left globus pallidus and right thalamus in the ex-MDMA users than in the control subjects. After registration in the same orientation (in six control subjects and two ex-MDMA users), an unpaired Student's t-test was performed on each voxel of generated rCBV maps, revealing significant differences in the colored regions (yellow, P < .025; red, P < .005). Images were obtained at intervals of 1202 milliseconds (800/54) before, during, and after injection of contrast agent
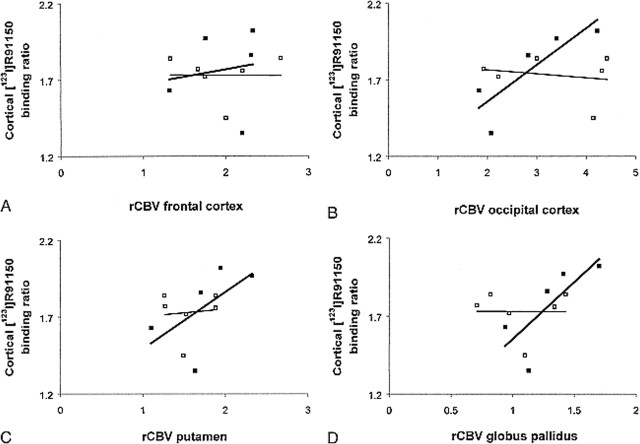
Correlations between 5-HT2 Receptor Densities and rCBV Values
In MDMA users, but not in control subjects, a significant positive correlation was found between cortical 5-HT2A receptor binding ratios and rCBV values in the globus pallidus and occipital cortex (in control subjects, [rho] = −.12 and −.06, respectively, and P = .74 and .91, respectively; in MDMA users, [rho] = .90 and .90, respectively, and P = .04 and .04, respectively) (Fig 4). The covariance effects of age, sex, and extent of previous MDMA use were not significant in the globus pallidus (P = .89, P = .74, and P = .18, respectively) or in the occipital cortex (P = .15, P = .11, and P = .08, respectively).
fig 4.
A–D, Cortical [123I]R91150 binding versus rCBV values in specific brain regions. Open square, control subjects; solid square, MDMA users. Each symbol represents an individual subject
Discussion
Data obtained in MDMA-treated rats have shown down-regulation of 5-HT2 receptors until several weeks after treatment, owing to high levels of synaptic 5-HT (18). Other studies have also shown that 5-HT release leads to a compensatory down-regulation of postsynaptic 5-HT2 receptors (24), whereas 5-HT depletion leads to an up-regulation of 5-HT2A receptors (25). Interestingly, in this study, we observed a significant lower cortical [123I]R91150 binding to 5-HT2A receptors in the MDMA group as compared with control subjects and ex-MDMA users. This finding suggests down-regulation of 5-HT2A receptors. MDMA is an amphetamine derivative, which induces release of 5-HT from serotonergic neurons (13, 26). The presently observed low cortical 5-HT2A receptor density in the MDMA group therefore suggests down-regulation due to MDMA-induced 5-HT release.
In contrast, the high binding of [123I]R91150 in the ex-MDMA group (although not statistically significant) suggests an up-regulation of postsynaptic 5-HT2A receptors due to MDMA-induced 5-HT depletion. It is known that abuse of MDMA leads eventually to loss of serotonergic neurons (27). For example, cortical 5-HT levels in MDMA-treated monkeys were still significantly reduced 13 months after treatment (27). Thus, the presently observed high [123I]R91150 binding may be the result of low synaptic 5-HT, possibly caused by loss of 5-HT neurons due to previous MDMA use. In a recent study it was shown that MDMA causes loss of 5-HT neurons not only in animals but in humans as well (28). Therefore, it could be hypothesized that in the ex-MDMA group loss of 5-HT neurons resulted in low synaptic 5-HT levels, leading to up-regulation of 5-HT2 receptors. The SPECT results obtained in the present study indicate the necessity for, and would probably justify, repeated 5-HT2 receptor studies within MDMA users.
In MDMA users, but not in control subjects, we found a significant positive correlation between cortical 5-HT2A receptor densities (measured with [123I]R91150 SPECT) and rCBV values (measured with dynamic MR imaging) in the occipital cortex and globus pallidus. Interestingly, several studies have pointed out that necrosis of the globus pallidus was the most striking neuropathologic change in postmortem material of MDMA users (9, 10). The globus pallidus is an area rich in 5-HT terminals. It is thought that local release of 5-HT, as induced after recent intake of MDMA, leads to prolonged vasospasm and necrosis of the globus pallidus (2, 9, 10), possibly because of stimulation of 5-HT receptors situated on microvessels by 5-HT. In addition, several studies have described cortical cerebral vascular accidents after MDMA use (1–8). The occipital cortex is another brain area rich in 5-HT–releasing neurons and 5-HT2A receptors (29). It has been shown that the occipital cortex is particularly sensitive to 5-HT neuronal injury, since MDMA-treated monkeys exhibited the most severe 5-HT depletion in the occipital cortex (27).
The presently observed correlation between cortical 5-HT2A receptor availability and rCBV values in the occipital cortex and globus pallidus in MDMA users but not in control subjects suggests that 5-HT2A receptors are involved in the pathogenesis of MDMA-induced abnormal vascular reactions, possibly leading to cerebrovascular accidents. It is known that 5-HT2A receptors play a key role in the regulation of brain microcirculation, since they are located on brain microvessels (11, 12). For years, 5-HT2 antagonists have proved effective in preventing migraine headache (30). It is thought that stimulation of 5-HT2A receptors by 5-HT mediates cerebral vasoconstriction. However, vasodilatations have also been observed (11). The short-term effect of MDMA involves excessive 5-HT release and stimulation of 5-HT2A receptors, leading to vasoconstriction. In line with this, we found that recent MDMA users had a significantly lower density of cortical 5-HT2A receptors (down-regulation due to high synaptic 5-HT levels) and lower rCBV values (vasoconstriction) in the occipital cortex and globus pallidus than did former users and nonusers of MDMA. On the other hand, former (ex-) MDMA users had a high density of cortical 5-HT2A receptors (up-regulation due to 5-HT depletion) and high rCBV values (vasodilatation) (as illustrated in Fig 3). In such a 5-HT deprived system, 5-HT2A receptors are not sufficiently stimulated, thus leading to vasodilatation instead of vasoconstriction. These findings suggest that MDMA users are susceptible to cerebrovascular accidents, resulting from vasoconstriction in recent users and from vasodilatation in former users.
The ratio of approximately 2.5 obtained in this study between cortical gray and white matter rCBV values in control subjects correlates well with the findings in other rCBV mapping MR imaging studies (22, 31). In addition, the ratios for [123I]R91150 binding obtained in control subjects are wholly consistent with those of other studies (14, 19).
Several potential limitations of the current study should be mentioned. First, as with all retrospective studies, there is a possibility that preexisting differences between MDMA users and nonusers underlie differences in 5-HT2A receptor densities and rCBV. Thus, people with low 5-HT2A receptor densities may be predisposed to use MDMA and to have low occipital and pallidal rCBV values. Second, the sample size was small. Nevertheless, 5-HT2A receptor densities represent unequivocal values and these data do provide useful preliminary evidence of the relationship between 5-HT2A receptor densities and rCBV values as revealed by SPECT and MR imaging. Furthermore, despite the known presence of 5-HT2A receptors in the globus pallidus, as demonstrated in both in vitro and in vivo studies (32, 33), in cortical regions, 5-HT2A receptor densities are about 10 times higher than in the basal ganglia (33); therefore, it is difficult to quantify 5-HT2A receptor densities reliably with SPECT. In a recent study it was shown that, in MDMA users, brain 5-HT transporter densities were globally decreased (28). It can be expected, therefore, that the extent of alterations in cortical 5-HT2A receptor densities, observed in the present study, reflect those in the globus pallidus. Third, in the present study, more women than men were included in the control group. Since there is evidence from animal experiments that 5-HT2 receptor density is higher in the brains of female animals than in male animals, the observed difference between control subjects and MDMA users may be a function of the larger number of women in the control group. However, a recent [123I]R91150 SPECT study, performed in healthy human subjects, showed no influence of sex on [123I]R91150 binding (34). Finally, all participants in the MDMA group in our study reported that they had abstained from use of MDMA or other psychoactive drugs for at least 1 week before the study. Although most of the MDMA users had experimented with other recreational drugs (mainly, alcohol and cannabis), none was a known 5-HT neurotoxin in humans; therefore, none was likely to account for changes in [123I]-5-I-R91150 binding to 5-HT2A receptors.
Conclusion
Our findings provide new support for the existence of a relationship between 5-HT2 receptor densities and rCBV values in specific regions of the brain. Taken in conjunction with the clinical data from other published studies and historical findings of cerebrovascular accidents in MDMA users, one may infer the existence of a relationship between the serotonergic system and MDMA-induced cerebrovascular accidents. Our data suggest a trend in which MDMA users may be susceptible to abnormal vascular reactions, induced by alterations in the 5-HT system, which eventually predispose the individual to cerebrovascular accidents. Additional studies and converging lines of evidence are needed to delineate better the potential of MDMA to induce cerebrovascular accidents in humans by altering the 5-HT system. Our observations, in accord with other (case) reports, indicate that people who use MDMA are unwittingly putting themselves at risk for developing not only neuronal 5-HT brain injury but cerebrovascular accidents as well. Furthermore, this study indicates that the putative relationship between cortical 5-HT2A receptor densities and rCBV values in the occipital cortex and globus pallidus may suggest a target for prevention and treatment in the form of selective 5-HT receptor agents in patients experiencing abnormal vascular reactions after MDMA use.
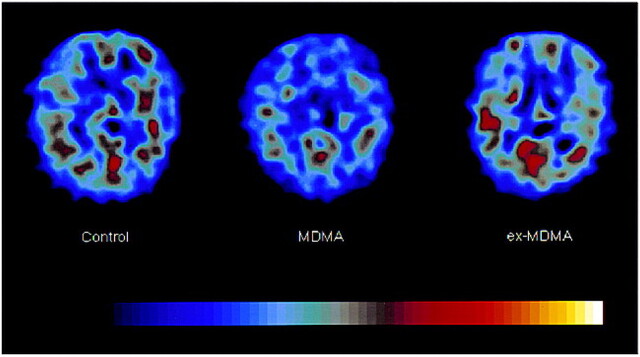
fig 2.
[123I]R91150 SPECT images in a control subject, a recent MDMA user, and an ex-MDMA user. Transverse slices from the brain at the level of the basal ganglia, approximately 3 cm above the orbitomeatal line. In the three images, the level of [123I]R91150 activity is color-coded from low (black) to high (white) and scaled to the maximum in the slice obtained in the control subject. The three images are representative of the three groups: in the control subject, there is normal [123I]R91150 binding, in the recent MDMA user, low [123I]R91150 binding, and in the ex-MDMA user, high [123I]R91150 binding
Acknowledgments
We thank Kora de Bruin and Ruud Smit for their technical assistance with the SPECT and MR imaging studies.
Footnotes
Presented in part at the annual meeting of the European Association of Nuclear Medicine, Barcelona, October 1999.
Address reprint requests to L. Reneman, MD, Department of Nuclear Medicine, F2N, Academic Medical Center, Meibergdreef 9, 1105 AZ Amsterdam, the Netherlands.
References
- 1.Hanyu S, Ikeguchi K, Imai H, Imai N, Yoshida M. Cerebral infarction is associated with 3,4-methylenedioxymethamphetamine (“Ecstasy”) abuse. Eur Neurol 1995;35:173. [DOI] [PubMed] [Google Scholar]
- 2.Henry JA, Jeffreys KJ, Dawling S. Toxicity and deaths from 3,4-methylenedioxymethamphetamine (“Ecstasy”). Lancet 1992;340:384-387 [DOI] [PubMed] [Google Scholar]
- 3.Harries DP, De Silva R. “Ecstasy” and intracerebral haemorrhage. Scott Med J 1992;37:150-152 [DOI] [PubMed] [Google Scholar]
- 4.De Silva RN, Harries DP. Misuse of ecstasy. BMJ 1992;305:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henry JA. Ecstasy and the dance of death. BMJ 1992;305:5-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gledhill JA, Moore DF, Bell D, Henry JA. Subarachnoid haemorrhage associated with MDMA abuse. J Neurol Neurosurg Psychiatry 1993;56:1036-1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes JC, McCabe M, Evans RJ. Intracranial haemorrhage associated with ingestion of “ecstasy.”. Arch Emerg Med 1993;10:372-374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teggin AF. Ecstasy: a dangerous drug. S Afr Med J 1992;101:431-432 [PubMed] [Google Scholar]
- 9.Squier MV, Jalloh S, Hilton-Jones D, Series H. Death after ecstasy ingestion: neuropathological findings. J Neurol Neurosurg Psychiatry 1995;50:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spatt J, Glawar B, Mamoli B. A pure amnestic syndrome after MDMA (“ecstasy”) ingestion. J Neurol Neurosurg Psychiatry 1997;62:418-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol 1996;50:335-362 [DOI] [PubMed] [Google Scholar]
- 12.Parsons AA. 5-HT receptors in human and animal cerebrovasculature. Trends Pharmacol Sci 1991;12:310-315 [DOI] [PubMed] [Google Scholar]
- 13.Green AR, Cross AJ, Goodwin GM. Review of the pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA or “Ecstasy”). Psychopharmacology 1995;114:247-260 [DOI] [PubMed] [Google Scholar]
- 14.Busatto GF, Pilowsky LS, Costa DC, et al. Initial evaluation of 123I-5-I-R91150, a selective 5-HT2A ligand for single-photon emission tomography, in healthy human subjects. Eur J Nucl Med 1997;29:119-124 [DOI] [PubMed] [Google Scholar]
- 15.Rosen BR, Belliveau JW, Aronen HJ, et al. Susceptibility contrast imaging of cerebral blood volume: human experience. Magn Reson Med 1991;22:293-299 [DOI] [PubMed] [Google Scholar]
- 16.Belliveau JW, Rosen BR, Kantor HL, et al. Functional cerebral imaging by susceptibility-contrast NMR. Magn Reson Med 1990;19:538-546 [DOI] [PubMed] [Google Scholar]
- 17.Kaufman MJ, Levin JM, Maas LC, et al. Cocaine decreases relative cerebral blood volume in humans: a dynamic susceptibility contrast magnetic resonance imaging study. Psychopharmacology 1998;138:76-81 [DOI] [PubMed] [Google Scholar]
- 18.Scheffel U, Lever JR, Stathis M, Ricaurte GA. Repeated administration of MDMA causes transient down-regulation of serotonin 5-HT2 receptors. Neuropharmacology 1992;31:881-893 [DOI] [PubMed] [Google Scholar]
- 19.Travis MJ, Busatto GF, Pilowsky LS, et al. 5-HT2A receptor blockade in patients with schizophrenia treated with risperidone or clozapine: a SPECT study using the novel 5-HT2A ligand 123I-5-I-R-91150. Br J Psychiatry 1998;173:236-241 [DOI] [PubMed] [Google Scholar]
- 20.Ostergaard L, Weiskoff RM, Chesler DA, Gyldensted C, Rosen B. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages, I: mathematical approach and statistical analysis. Magn Reson Med 1996;36:715-725 [DOI] [PubMed] [Google Scholar]
- 21.Rosen B, Belliveau J, Chien D. Perfusion imaging by nuclear magnetic resonance. Magn Reson Q 1989;5:263-281 [PubMed] [Google Scholar]
- 22.Aronen HJ, Gazit IE, Louis DN, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 1994;191:41-51 [DOI] [PubMed] [Google Scholar]
- 23.Aronen HJ, Glass J, Pardo FS, et al. Echo-planar MR cerebral blood volume mapping of gliomas: clinical utility. Acta Radiol 1995;36:520-528 [PubMed] [Google Scholar]
- 24.Peroutka SJ, Snyder SH. Long-term antidepressant treatment decreases spiroperidol-labeled serotonin receptor binding. Science 1980;210:88-90 [DOI] [PubMed] [Google Scholar]
- 25.Heal DJ, Philpot J, Molyneux SG, Metz A. Intracerebroventricular administration of 5,7-dihydroxytryptamine to mice increases both head-twitch response and the number of cortical 5-HT2 receptors. Neuropharmacology 1985;24:1201-1205 [DOI] [PubMed] [Google Scholar]
- 26.White SR, Obradovic T, Imel KM, Wheaton MJ. The effects of methylenedioxymethamphetamine (MDMA, “Ecstasy”) on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol 1996;49:455-479 [DOI] [PubMed] [Google Scholar]
- 27.Scheffel U, Szabo Z, Mathews WB, et al. In vivo detection of short- and long-term MDMA neurotoxicity: a positron emission tomography study in the living baboon brain. Synapse 1998;29:183-192 [DOI] [PubMed] [Google Scholar]
- 28.McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet 1998;352:1433-1437 [DOI] [PubMed] [Google Scholar]
- 29.Gross-Isseroff R, Salama D, Israeli M, Biegon A. Autoradiographic analysis of age-dependent changes in serotonin 5-HT2 receptors of the human brain postmortem. Brain Res 1990;519:223-227 [DOI] [PubMed] [Google Scholar]
- 30.Mylecharane EJ. 5-HT2 receptor antagonists and migraine therapy. J Neurol 1991;238:S45-S52 [DOI] [PubMed] [Google Scholar]
- 31.Lammertsma AA, Wise RJS, Cox TcS, Thomas DGT, Jones T. Measurement of blood flow, oxygen utilisation, oxygen extraction ratio, and fractional blood volume in human brain tumours and surrounding oedematous tissue. Br J Radiol 1985;58:725-734 [DOI] [PubMed] [Google Scholar]
- 32.Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain, IV: autoradiographic mapping of serotonin-2 receptors. Neuroscience 1987;21:123-139 [DOI] [PubMed] [Google Scholar]
- 33.Schotte A, Maloteaux JM, Laduron PM. Characterization and regional distribution of serotonin S2-receptors in human brain. Brain Res 1983;276:231-235 [DOI] [PubMed] [Google Scholar]
- 34.Baeken C, D'Haenen H, Flamen P, et al. 123I-5-R91150, a new single-photon emission tomography ligand for 5-HT2A receptors: influence of age and gender in healthy human subjects. Eur J Nucl Med 1998;25:1617-1622 [DOI] [PubMed] [Google Scholar]