Abstract
BACKGROUND AND PURPOSE: Because the presence of cervical metastasis is one of the factors influencing the outcome of patients with carcinoma of the head and neck, its early detection is potentially very important. The purpose of this study was to evaluate the characteristic changes of cervical metastasis revealed by sonography during follow-up and to assess an adequate interval for follow-up sonography of the neck among patients with tongue cancer.
METHODS: Forty-three of 44 consecutive patients with squamous cell carcinoma of the tongue, who had undergone interstitial brachytherapy, were examined with sonography of the neck during the posttherapeutic follow-up period.
RESULTS: Seventeen cervical lymph node metastases were found in 12 of 43 patients during follow-up. Of these 17 cervical metastases, 16 (94.1%) were accurately diagnosed and one (5.9%) was misdiagnosed as nonmetastatic based on sonographic findings. Sonography of the neck performed in seven patients at an interval of less than 1 month since the last follow-up imaging showed 9 (90.0%) of 10 metastases increased by up to 2 mm in short-axis diameter. Five patients who were followed up at an interval of more than 1 month since the last follow-up imaging had seven metastases increase by 3 to 8 mm in short-axis diameter or a change of echogenicity in the internal structure of lymph nodes or both. Pathologic examinations showed extranodal spread in 3 (42.9%) of these 7 metastases.
CONCLUSION: Changes both in size and internal echogenicity can occur as quickly as 2 to 4 weeks between sonographic examinations. Therefore, in high-risk patients, or in those with suspicious sonographic findings, short-interval follow-up sonographic examinations are recommended at least during the first posttherapeutic year. Our findings suggest that follow-up sonography of the neck should be performed monthly, at least during the first posttherapeutic year.
The purpose of posttreatment follow-up imaging of malignant tumor is for early detection of local recurrence, a new primary tumor, and cervical and distant metastasis. Routine follow-up is also required for head and neck cancer (1, 2). Marchant et al (1) reported that 73% of physicians actively practicing head and neck surgery saw their patients monthly during the first postoperative year. Eighty-one percent of them ordered CT scans of the head and neck region for patient symptoms only, and 8% of the responding surgeons performed baseline neck CTs during the immediate postoperative period (1). Cervical lymph node metastasis, especially extranodal metastasis, is one of the factors influencing patient outcome. Its early detection within a short interval of posttherapeutic follow-up is desirable to ensure that additional therapy is not delayed (3–5). Therefore, cervical lymph nodes should also be examined carefully with not only palpation but also diagnostic imaging procedures such as CT, sonography, and MR imaging (6–8). Sonography is especially useful for monitoring the status of cervical lymph nodes (9, 10). Snow (11) has pointed out the necessity of sonography at each follow-up time; however, few have attempted to study changes in sonographic findings of cervical lymph node metastasis during follow-up and to identify an adequate period in which follow-up sonography should be performed for patients with head and neck cancer.
We have performed routine follow-up sonography for patients with tongue cancer, who underwent only primary treatment by radiotherapy. The routine follow-up included recent medical history, regional examination, chest radiography, and sonography of the neck. We sought to evaluate characteristic changes of cervical lymph node metastasis that had been diagnosed as nonmetastatic, based on sonographic findings at pretreatment, and to determine the proper interval for follow-up sonography of the neck for patients with tongue cancer.
Methods
Forty-four consecutive patients who were diagnosed as having T1-2N0M0 tongue squamous cell carcinoma at pretreatment, and were treated by interstitial brachytherapy between January 1995 and December 1996, were reviewed. No radiation was given to the nodes during follow-up. Forty-three patients underwent follow-up sonography of the neck. Twenty-three men and 20 women (age range, 33 to 88 years [mean age, 61.2 years]) were studied prospectively. Forty-three patients with 565 cervical lymph nodes were followed up by sonography. Informed consent was obtained from all patients.
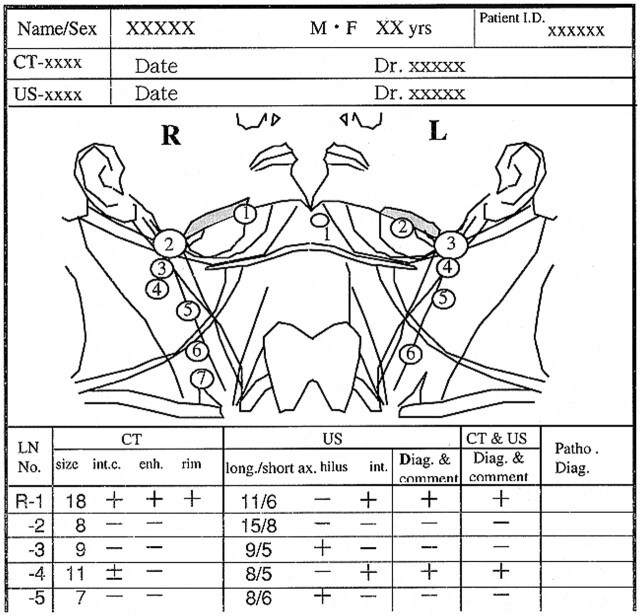
Sonography was performed with the high-resolution real-time RT-2600 liner scanner (Yokogawa Medical Co., Tokyo, Japan) with a 7.5-MHz transducer with a stand-off pad (Kitecko; 3M Health Care Limited, Texas, USA). Sonography was done by B-mode, and the scan obtained by the one radiologist (K.Y). Sonograms were interpreted by the same radiologist (K.Y). All lymph nodes were documented with a laser image scanner (Li-10; Konica, Tokyo, Japan) in both transverse and longitudinal nodal planes. The internal structures of lymph nodes, such as the hilar (homogeneous echogenic structure) and internal echoes, were evaluated. In addition, The short-axis diameter of lymph nodes was measured. The size, position, and internal characteristics of the cervical lymph nodes were recorded on topographic illustrations (Fig 1). First, numbering was made of the pretreatment examination in 26 patients and of first posttreatment examination of 17 patients in whom metastasis had been ruled out at other hospitals. Each subsequent sonogram was compared with the last sonographic finding throughout the follow-up period by using topographic illustrations.
fig 1.
An example of a report of a cervical lymph node with topographic illustrations
Of 43 patients who underwent follow-up sonography, 12 (27.9%) who were suspicious for metastatic disease based on follow-up sonographic findings underwent ipsilateral neck dissection. All lymph nodes excised at surgery were examined histopathologically on serially cut tissue. The remaining 31 patients were not suspicious for metastatic disease based on careful clinical examination and diagnostic imaging procedures, such as sonography and CT, throughout at least 24 months' follow-up period. Therefore, they did not undergo neck dissection; we determined that lymph nodes in these 31 patients were not metastases and therefore these individuals should not participate in this study.
Results
In 12 (27.9%) of 43 patients who were followed up with sonography, 16 lymph nodes in 12 necks were diagnosed as metastases. In these 12 patients, neck dissection and subsequent pathologic examination revealed metastasis in 17 lymph nodes and nonmetastasis in 64 lymph nodes. All 16 lymph nodes that had been diagnosed as metastasis based on follow-up sonographic findings were pathologically confirmed as metastasis. One (5.9%) of 17 metastases was incorrectly assessed as a nonmetastasis based on sonographic findings. All 64 lymph nodes that had been diagnosed as nonmetastasis based on follow-up sonography were pathologically confirmed as nonmetastasis. In the remaining 31 (72.1%) patients who did not undergo neck dissection, there was no evidence of cervical lymph node metastasis during at least 24 months' follow-up. Therefore, we concluded that these 31 patients did not have metastasis. No nonmetastatic lymph nodes in these 31 patients (484 lymph nodes) increased by more than 2 mm in short-axis diameter or showed echogenicity during follow-up. True-positive and true-negative findings were 100% and 100%, respectively. False-positive and false-negative findings were 0% and 5.9%, respectively.
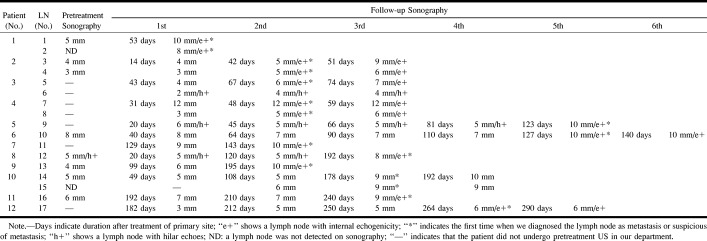
Changes in sonographic data of cervical lymph node metastasis obtained from patients 1 to 12 during the follow-up period are summarized in the Table. Two lymph nodes in patient 1 were diagnosed as metastatic, because the first follow-up sonographic examination showed internal echogenicity. Of these two lymph nodes, a smaller one, which had not been revealed by sonography before treatment, was first detected at the first follow-up examination 53 days after primary treatment, whereas the other node increased by 5 mm in short-axis diameter since pretreatment sonography. Because two lymph nodes of patient 2 had been suspicious for metastasis based on the second follow-up sonographic examination, the next examination was performed shortly thereafter. Consequently, one of these two lymph nodes increased by 4 mm in short-axis diameter only 9 days after the second follow-up sonographic examination (Fig 2). Pathologic examination confirmed extranodal spread of metastasis. One of two metastatic nodes in patient 3 increased by 2 mm in short-axis diameter. This finding was accompanied by the appearance of internal echogenicity 24 days after the first follow-up sonographic examination, whereas the other had normal hilar echoes in spite of a similar increase in size (2 mm, 24 days after the first follow-up sonographic examination). Therefore, the latter node was misdiagnosed as benign. Two lymph nodes in patient 4 were diagnosed as metastases because of the appearance of internal echogenicity revealed by the second follow-up sonographic examination. In patient 5, no change in the size or hilar appearance was observed. Sonographic examination performed at a long interval (42 days after the fourth follow-up sonogram was obtained), however, showed an increase in short-axis diameter by 5 mm, accompanied by developing internal echogenicity (Fig 3). Pathologically, extranodal spread metastasis was confirmed. The metastatic lymph node in patient 6 increased by 3 mm in short-axis diameter and developed internal echogenicity only 17 days after the fourth follow-up sonographic examination. In patient 7, the second follow-up sonographic examination showed the appearance of internal echogenicity, consistent with lymph node metastasis. In patient 8 and 9, the lymph nodes increased by 3 or 4 mm in short-axis diameter, and also showed internal echogenicity.
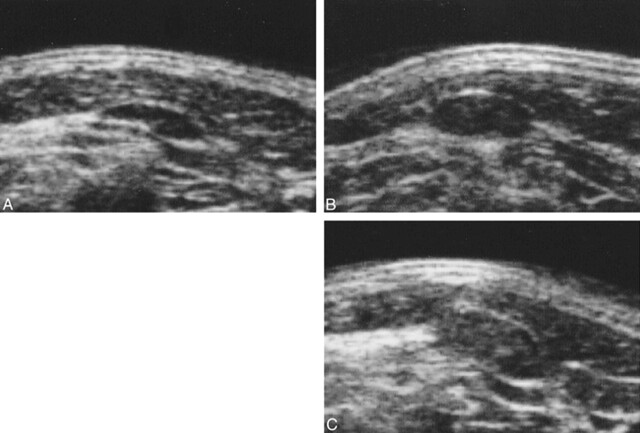
fig 2.
Follow-up sonogram of lymph node in patient 2. A, The first follow-up sonogram. The lymph node measures 3 mm in short-axis diameter. B, The second follow-up sonogram (28 days after the first follow-up sonogram was obtained). Short-axis diameter of the lymph node has increased by 2 mm. Slight, internal echogenicity, suspicious for metastasis, is visible. C, The third follow-up sonogram (9 days after the second follow-up sonogram was obtained). Short-axis diameter of the lymph node has increased by 1 mm. Marked internal echogenicity is increased, and the boundary of the node is unclear, suggesting extranodal extension of disease. Pathologic examination of this lymph node confirmed metastasis, with extranodal extension of disease.
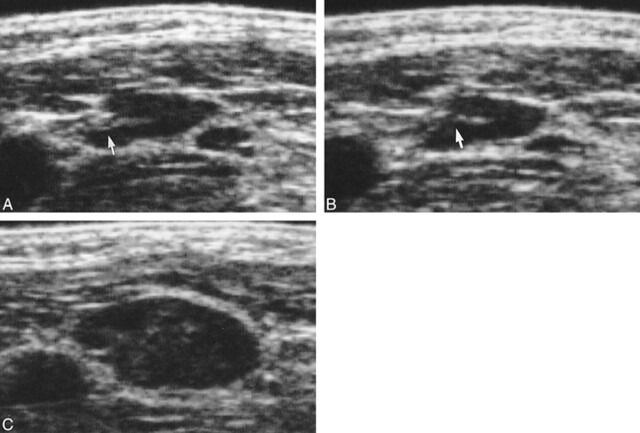
fig 3.
Follow-up sonograms of the lymph node in patient 5. A, The third follow-up sonogram. The lymph node measures 5 mm in short-axis diameter, with normal hilar echoes (arrow). B, The fourth follow-up sonogram (35 days after the third follow-up sonogram was obtained). The size of the lymph node has not changed, and normal hilar echoes are also observed. In retrospect, we should have noticed that the hilar echoes were slightly less distinct compared with those revealed by the last sonogram (arrow), and should have performed sonography at a shorter interval after performing the third follow-up examination. C, The fifth follow-up sonogram (42 days after the fourth follow-up sonogram was obtained). The lymph node has increased by 5 mm in short-axis diameter. Internal echogenicity is also observed, and the normal hilar echoes are no longer visible. Pathologic anlaysis of this lymph node confirmed metastasis with extranodal extension of disease.
Pathologically, extranodal spread metastases were found. Although internal echogenicity was not observed in patient 10, two lymph nodes were diagnosed as metastasis, because the third follow-up sonographic examination showed an increase by 3 or 4 mm in short-axis diameter. The lymph node in patient 11 increased by 2 mm in short-axis diameter, with the appearance of internal echogenicity on the third follow-up sonogram. The fourth follow-up sonographic examination revealed metastasis in the lymph node of patient 12 because of appearance of echogenicity.
In seven patients (patients 2–4, 6, 7, 11, 12) who underwent follow-up sonography at an interval of less than 1 month (9 to 30 days) since the last follow-up imaging examination, nine (90.0%) of 10 metastases increased by 0 to 2 mm in short-axis diameter. Pathologic examinations showed extranodular spread in one (10.0%) of these 10 metastases. In five patients (patient 1, 5, 8–10) who underwent follow-up sonography at an interval of more than 1 month (42 to 96 days) since the last sonogram was obtained, all seven metastases increased by 3 mm to 8 mm in the short-axis diameter or changed in appearance from hilar echoes to internal echogenicity or both. Pathologic examinations showed extranodular spread in three (42.9%) of these seven metastases.
Discussion
Sonography is a noninvasive and less expensive diagnostic procedure than is MR imaging or CT. Sonography has been used to monitor cervical lymph nodes in cancer patients during follow-up, as well as to play a role in cancer staging and therapeutic planning (9, 10). In this study, patients with tongue cancer, who received treatment with interstitial brachytherapy, were followed up by sonography to detect cervical lymph node metastasis. Many studies have focused on diagnostic sonographic criteria such as the size, shape, and internal echogenicity for cervical metastasis (12–18). It has been reported that the presence of hilar echoes may suggest normal nodes (18, 19). In this study, the appearance of internal echogenicity or an increase in short-axis diameter was used to suggest metastatic disease. In 10 of 17 lymph nodes in our series, sonography accurately revealed metastasis before enlargement to 10 mm or more in short-axis diameter. One node, however, was misdiagnosed as benign because of presence of hilar echoes. Our previous study showed that hilus was observed in about 7% of metastases (20). In this case, an increase in short-axis diameter should have been taken into consideration. Furthermore, careful attention should be paid to slight changes in hilar echoes (patient 5 [Fig 3]). In addition, Vassallo et al (12) asserted that the presence of hilus narrowing should be regarded as suspicious for malignancy.
Usually we examine patients treated with radiotherapy every 2 weeks during the 1st posttherapeutic year, monthly during the 2nd year, every 2 months during the 3rd year, every 3 months during the 4th year, every 6 months during the 5th year and every year thereafter. Our follow-up is made on a more frequent basis than what we have observed in other reports (1, 2, 11). In this study, however, sonography was not performed during each follow-up visit. It was performed when the sonographic machine was available for examination. In our series, nine of 10 metastatic nodes were detected in seven patients who underwent sonography of the neck at an interval of less than 1 month since their last follow-up imaging examination. These nodes increased up to 2 mm in short-axis diameter. Pathologic examination revealed extranodal metastasis in one of these lymph nodes. In contrast, all seven metastases in five patients who were followed up at an interval of more than 1 month after the last sonographic examination increased by 3 to 8 mm in short-axis diameter. In some of them, alternations in the internal structures, loss of the hilar echoes, and increased internal echogenicity of lymph node were seen. Three of these seven lymph nodes had extranodal metastases that were pathologically confirmed. These results suggest that metastasis may abruptly change the size or internal structure of lymph nodes, even within a 1-month period. Thus, it would be better to perform follow-up sonography of the neck at least once a month. It is, however, impractical to perform sonography once a month for a long follow-up period. Because all metastases in our study developed within the first year after treatment, we suggest that sonography be performed monthly at least during the first posttherapeutic year, when cervical nodal metastases are most likely to occur (21–23), in order to detect metastatic disease before extranodal invasion occurs. Tongue cancer is reported to show the highest incidence of cervical occult metastasis among oral cancers (23–25). If suspicious evidence of metastasis such as a change in short-axis diameter or internal structure of the lymph node is detected, follow-up sonographic examination should be done even earlier, perhaps at 2-week intervals.
Conclusion
Our results suggest that sonographic findings of metastasis, such as a change in lymph node size or internal echogenicity, may change relatively quickly, even within a 2- to 4-week period. Therefore, follow-up sonography of the neck should be performed monthly, at least during the first posttherapeutic year, especially for patients at high risk for nodal diseases.
The changes of short-axis diameter and internal structures of metastatic lymph nodes in follow-up sonography
Footnotes
Address reprint requests to Kenji Yuasa, Department of Oral and Maxillofacial Radiology, Faculty of Dentistry, Kyushu University, Maidashi 3-1-1, Higashi-ku, Fukuoka 812-8582, Japan.
References
- 1.Marchant FE, Lowry LD, Moffitt JJ, Sabbagh R. Current national trends in the posttreatment follow-up of patients with squamous cell carcinoma of the head and neck. Am J Otolaryngol 1993;14:88-93 [DOI] [PubMed] [Google Scholar]
- 2.de Visscher AV, Manni JJ. Routine long-term follow-up in patients treated with curative intent for squamous cell carcinoma of the larynx, pharynx, and oral cavity. Does it make sense? Arch Otolaryngol Head Neck Surg 1994;120:934-939 [DOI] [PubMed] [Google Scholar]
- 3.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer 1993;71:452-456 [DOI] [PubMed] [Google Scholar]
- 4.Parrsons JT, Mendenhall WM, Springer SP, Cassissi NJ, Million RR. An analysis of factors influencing the outcome of postoperative irradiation for squamous cell carcinoma of the oral cavity. Int J Radiat Oncol Biol Phys 1997;39:137-148 [DOI] [PubMed] [Google Scholar]
- 5.Kalnins IK, Leonard AG, Sako K, Razack MS, Shedd DP. Correlation between prognosis and degree of lymph node involvement in carcinoma of the oral cavity. Am J Surg 1977;134:450-454 [DOI] [PubMed] [Google Scholar]
- 6.Tachimori Y, Kato H, Watanabe H, Yamaguchi H. Neck ultrasonography for thoratic esophageal carcinoma. Ann Thorac Surg 1994;57:1180-1183 [DOI] [PubMed] [Google Scholar]
- 7.Jone DG, Wiliams SR, Ahuja A, et al. Palpation compared with ultrasound in the assessment of malignant cervical lymph nodes. J Laryngol Otol 1993;107:821-823 [DOI] [PubMed] [Google Scholar]
- 8.Battenburg RJ, Rongen RJ, Lameris JS, Harthoon M, Verwoerd CDA, Knegt P. Metastatic neck disease. Palpation vs ultrasound examination. Arch Otolaryngol Head Neck Surg 1989;115:689-690 [DOI] [PubMed] [Google Scholar]
- 9.Antonelli A, Miccoli P, Ferdeghini M, et al. Role of neck ultrasonography in the follow-up of patients operated on for thyroid cancer. Thyroid 1995;5:25-28 [DOI] [PubMed] [Google Scholar]
- 10.Westhofen M. Ultrasound B-scans in the follow-up of head and neck tumors. Head Neck Surg 1987;9:272-278 [DOI] [PubMed] [Google Scholar]
- 11.Snow GB. Follow-up in patients treated for head and neck cancer: how frequent, how thorough and for how long? Eur J Cancer 1992;28:315-316 [DOI] [PubMed] [Google Scholar]
- 12.Vassallo P, Wernecke K, Roos N, Peters P. Differentiation of benign from malignant superficial lymphadenopathy: the role of high-resolution US. Radiology 1992;183:215-220 [DOI] [PubMed] [Google Scholar]
- 13.Bruneton JN, Roux P, Caramella E, Demard F, Vallicioni J, Chauvel P. Ear, nose, and throat cancer: Ultrasound diagnosis of metastasis to cervical lymph nodes. Radiology 1984;152:771-773 [DOI] [PubMed] [Google Scholar]
- 14.Sakai F, Kiyono K, Sone S, et al. Ultrasonic evaluation of cervical metastatic lymphadenopathy. J Ultrasound Med 1988;7:305-310 [DOI] [PubMed] [Google Scholar]
- 15.Steinkamp HJ, Cornehl M, Hosten N, Pegios W, Vogl T, Felix R. Cervical lymphadenopathy: Ratio of long- to short -axis diameter as a predictor of malignancy. Br J Radiol 1995;68:266-270 [DOI] [PubMed] [Google Scholar]
- 16.Tohnosu N, Onoda S, Isano K. Ultrasonographic evaluation of cervical lymph node metastases in esophageal cancer with special reference to the relationship between the short to long axis ratio (S/L) and the cancer content. J Clin Ultrasound 1989;17:101-106 [DOI] [PubMed] [Google Scholar]
- 17.Ahuja A, Ying M, Yang WT, Evans R, King W. The use of sonography in differentiating cervical lymphomatous lymph nodes from cervical metastatic lymph nodes. Clin Radiol 1996;51:186-190 [DOI] [PubMed] [Google Scholar]
- 18.Rubaltelli L, Proto E, Salmaso R, Bortoletto P, Candiani F, Cargol P. Sonography of abnormal lymph nodes in vitro: Correlation of sonographic and histologic findings. AJR Am J Roentgenol 1990;156:1241-1990 [DOI] [PubMed] [Google Scholar]
- 19.Sutton RT, Reading CC, Charboneau JW, James EM, Grant CS, Hay ID. US-guided biopsy of neck masses in postoperative management of patients with thyroid cancer. Radiology 1988;168:769-772 [DOI] [PubMed] [Google Scholar]
- 20.Yuasa K, Kawazu T, Kanda S. Cervical lymph nodes in oral cancer patients: CT and US study. In: Farman AG, ed. Advances in Maxillofacial Imaging. Amsterdam: Elservier Science B. V.; 1997: 239-244
- 21.Leemans CR, Tiwari R, Nauta JJ, van der Waal I, Snow GB. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer 1994;73:187-190 [DOI] [PubMed] [Google Scholar]
- 22.Kurokawa H, Murata T, Yamashita Y, et al. Clinicopathological evaluation of secondary cervical lymph node metastasis in oral squamous cell carcinoma. Jpn J Oral Maxillofac Surg 1997;43:661-666 [Google Scholar]
- 23.Takada K, Endo K, Nakamura K, Sakamoto T, Moroyama T, Yoshiga K. Clinicostatistical investigation on the cervical lymph node metastasis of oral malignant tumor. Jpn J Oral Maxillofac Surg 1988;34:872-878 [Google Scholar]
- 24.Cunningham MJ, Johnson JT, Myers EN, Schramm VL Jr, Thearle PB. Cervical lymph node metastasis after local excision of early squamous cell carcinoma of the oral cavity. Am J Surg 1986;152:361-366 [DOI] [PubMed] [Google Scholar]
- 25.DiTroia JF. Nodal metastasis and prognosis in carcinoma of the oral cavity. Otolaryngol Clin North Am 1972;5:333-342 [PubMed] [Google Scholar]