Abstract
BACKGROUND AND PURPOSE: The distribution of multiple sclerosis (MS) lesions in the brain follows a specific pattern, with most lesions in the periventricular regions and in the deep white matter; histopathologic studies have shown a perivenous distribution. The aim of this study was to illustrate these distribution patterns in vivo using high-resolution MR venography.
METHODS: Seventeen MS patients underwent MR imaging at 1.5 T. Venographic studies were obtained with a 3D gradient-echo technique. MS lesions were identified on T2-weighted images, and their shape, orientation, and location were compared with the venous anatomy on the venograms.
RESULTS: The use of contrast material facilitated the visualization of small veins and increased the number of veins seen. A total of 95 MS lesions could be identified on both the T2-weighted series and the venograms; a central vein was visible in all 43 periventricular lesions and in all but one of the 52 focal deep white matter lesions. The typical ovoid shape and orientation of the long axis of the MS lesions correlated well with the course of these veins.
CONCLUSION: With MR venography, the perivenous distribution of MS lesions in the brain can be visualized in vivo. The venous anatomy defines the typical form and orientation of these lesions.
Multiple sclerosis (MS) is an acquired demyelinating disease of unknown origin, manifested by episodic, (multi)focal neurologic symptoms. Although the sensitivity of MR imaging in depicting MS lesions in the brain is very high (1), its specificity is low. Specific features like the topography of lesions can increase the specificity. Juxtacortical lesions, irregular and confluent periventricular lesions, or lesions in the corpus callosum can be suggestive of MS. The morphology of lesions is also important; ovoid lesions and so-called Dawson fingers are typically found in MS (2–4). Postmortem studies have shown that this typical distribution and form of MS lesions in the brain can be explained by their perivenous location (2).
Recently, Reichenbach et al (5) showed that by using a flow-compensated, 3D RF-spoiled, gradient-echo sequence, small veins in the brain could be depicted. This method, referred to as MR venography, is based on the T2* shortening in deoxygenated blood, as compared with oxygenated blood, and on the concomitant developing phase difference between venous blood spins and tissue spins at long TEs. The aim of this study was to use MR venography to demonstrate in vivo that MS lesions have a perivenous distribution.
Methods
The study was performed on a 1.5-T whole-body scanner using a standard circularly polarized head coil. Seventeen patients (24–60 years old) with clinically definite MS (6) who were participating in a clinical trial gave their informed consent for the present study.
MR Techniques
Global shimming was performed to optimize the static field homogeneity before imaging. According to the trial protocol, a series of transverse noncontrast T1-weighted spin-echo (SE) images were acquired (625/12/2 [TR/TE/excitations]) followed by a dual-echo T2-weighted SE (2500/20–80/1) and T1-weighted SE series after administration of 0.2 mmol/kg gadopentetate dimeglumine. The imaginary line connecting the hypophysis and the fastigium of the fourth ventricle was used as the axial angulation of the slices (contiguous 3-mm slices with a pixel size of 1 × 1 mm). A region showing periventricular and/or focal MS lesions was selected on the T2-weighted SE series and rescanned using a transverse T2 turbo SE sequence (7000/112/2, voxel size = 0.65 × 0.49 × 2.00 mm, gap = 0.5 mm) with an axial angulation of 90° to the cerebral falx. This sequence was followed by transverse venography (one slab of 50 mm, 20 partitions), taking care that the 3D images from the venographic series were in the identical position as the T2 turbo SE images. Scan parameters were 67/50/1, with α = 20°, matrix = 288 × 512, and field of view = 188 × 250 mm, resulting in a voxel size of 0.65 × 0.49 × 2.50 mm. Acquisition time was 6 minutes 28 seconds per slab. The magnitude images were masked five times with the normalized phase mask to increase the visibility of venous structures (5, 7). In the first five patients, venography was performed before and after administration of contrast agent.
Image Analysis
In the analysis of the venograms, emphasis was placed on the visibility of veins, the effect of contrast material, and the location and orientation of MS lesions in relation to veins. Areas of artifacts in which veins could not be clearly recognized were identified so that lesions in such areas could be excluded from the analysis. MS lesions were considered those that were visible on the T2 series as discrete hyperintense areas and thereby clearly recognizable on the corresponding venogram.
Results
In the first five patients, venograms obtained with and without contrast agent proved that the use of contrast material facilitated the visibility of small veins and increased the number of veins visualized. Therefore, in the remaining 12 patients, only contrast-enhanced venograms were acquired. In two patients, motion artifacts influenced the visibility of small veins slightly, but did not interfere with the analysis of the venograms.
The venograms clearly showed the deep cerebral veins, including deep medullary veins running from the anterior part of the frontal lobe to drain into the septal veins, which join the internal cerebral vein at the foramen of Monro (Fig 1), and the thalamostriate vein, running forward at the junction of the thalamus and caudate nucleus, receiving the anterior terminal vein and numerous transverse caudate veins, and draining into the white matter of the frontoparietal region and the superior and anterior parts of the striate body (Fig 2). Because venography is an RF-spoiled gradient-echo technique, iron-containing structures, such as the red nucleus, the substantia nigra, and the globus pallidus, produce marked signal loss (Fig 3). Similarly, neither MS lesions nor (small) veins could be confidently identified in the parenchyma near the air-tissue interfaces around the sinuses or in the mastoid and infratentorial regions (Figs 1–3).
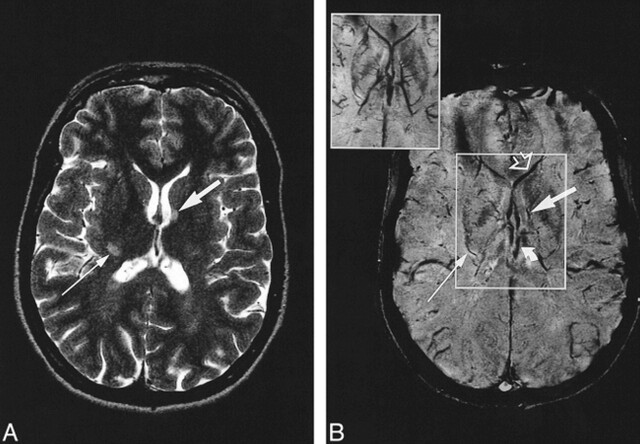
fig 1.
A and B, Axial T2-weighted MR image (7000/112/2) (A) at the level of the lateral ventricles and corresponding contrast-enhanced MR venogram (67/50/1) (B). The inset in B is the minimum intensity projection image calculated over 7.5 mm of the boxed area. The form and orientation of the left periventricular MS lesion (wide arrow) corresponds to the course of the longitudinal caudate vein. The lesion at the posterior limb of the right internal capsule (thin arrow) corresponds to the course of a striate vein. Curved arrow indicates internal cerebral vein; open arrow, septal vein
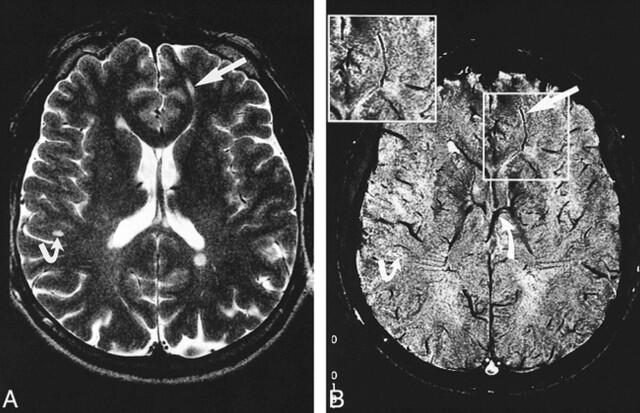
fig 2.
A and B, Axial T2-weighted MR image (7000/112/2) (A) at the level of the lateral ventricles and corresponding contrast-enhanced MR venogram (67/50/1) (B), with magnified image shown in boxed area. Periventricular lesions as well as a deep white matter lesion (straight arrow) and subcortical lesion (bent arrow) are demonstrated. The form and orientation of the lesions correspond to the course of their in-plane running central vein. The deep medullary vein running through the deep white matter lesion drains into the septal vein, and the caudate veins drain into the thalamostriate vein (curved arrow)
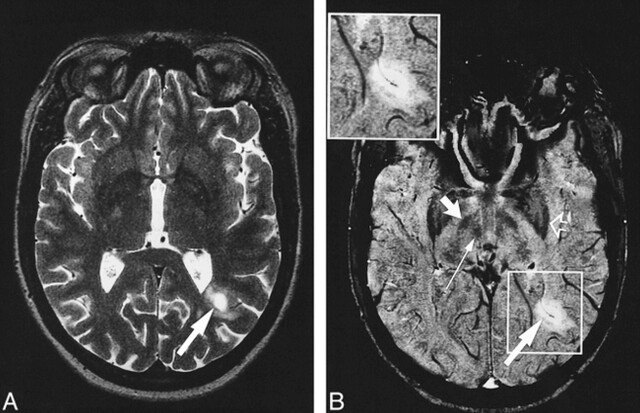
fig 3.
A and B, Axial T2-weighted image (7000/112/2) (A) and corresponding contrast-enhanced MR venogram (67/50/1) (B) with magnified image shown in boxed area. The lesion at the left occipital horn (long wide arrow) is hypointense, with ring enhancement visible on the T1-weighted SE image (not shown). The MR venogram shows a vein with its course corresponding to the form of the lesion. Note the susceptibility artifacts in the frontal regions and the low intensity of the globus pallidus (open arrow), substantia nigra (short wide arrow), and red nucleus (thin arrow)
A total of 95 MS lesions were identified that were clearly visible both on the T2-weighted series and on the venograms, with a frequency of two to 15 lesions per patient (mean, eight lesions per patient). Forty-three lesions had a periventricular location whereas 52 were nonperiventricular. The smallest diameter was 3 mm. Five of these nonperiventricular lesions (of which four were found in one patient) had a (sub)cortical location. Only in one strongly enhancing subcortical lesion did venography fail to depict a central vein. In all periventricular and deep white matter lesions, a vein could be seen coursing centrally through the lesion. Especially in periventricular lesions, more than one vein could often be identified within a lesion (Fig 2). The form and orientation of the lesions clearly followed the course of the veins running through these lesions. Ovoid lesions in the temporoparietal regions, with their long axis pointing perpendicular to the anteroposterior axis of the lateral ventricles, had a left to right–orientated vein coursing through it, as shown in Figure 2. Likewise, ovoid lesions in the frontal regions, with their axes parallel to the ventricles, were located around veins coursing in an anterior to posterior direction in the parenchyma (Fig 2), whereas periventricular MS lesions with an oblique orientation to the ventricles had obliquely running veins in their center. In one patient with a ring-shaped contrast-enhancing periventricular lesion, the venogram also clearly showed the obliquely running central vein within this active lesion (Fig 3). In images in which the long axis of the lesions was perpendicular to the imaging plane, a through-plane running vein in an MS lesion could be seen as a small, dark, centrally located dot that could be tracked to adjacent slices.
Discussion
The distribution and form of MS lesions have been well depicted on MR images and histopathologic studies, which have shown that the topography and morphology relate to the origin of lesions around a perivenous inflammation (3). To date, their relation to venules and small veins has been confirmed only by postmortem studies of CNS tissue (2, 3, 8, 9). To our knowledge, this perivenous location of MS lesions has not been demonstrated previously in vivo. We used the venographic technique described by Reichenbach et al (5, 7), which can depict veins in the brain with a diameter as small as 1 mm or less, to confirm that MS lesions have a perivenous location and that their form is determined by the location and orientation of veins. Among the 95 MS lesions identified, the venograms showed a vein running centrally in all but one subcortical lesion. The orientation and form of the MS lesions corresponded well with the course of this vein, confirming their perivenous origin. Veins in lesions as small as 3 mm could be identified. Consistent with the findings of Fog (2), a few lesions were found to be multivenous; that is, several veins could be seen within one lesion. In a microscopic study on the topography of MS lesions to elucidate their relationship to vessels, Fog demonstrated that out of 43 analyzed plaques, 39 followed one or two veins during their course through the white matter. Eleven of these 39 lesions were prolongations from perivascular lesions, so-called Dawson fingers. The course of periventricular plaques followed the course of central veins, branches of the subependymal venous plexus. The extension of the plaques depended on the ramification and the course of these branches. Lesions around the central veins in the white matter had a fusiform shape, with the greatest diameter of the lesion corresponding to the greatest diameter of the vein. Cortical and subcortical lesions had the form of flat cones, with one or two large veins located in their center. Using histochemical and immunocytochemical staining methods, Adams et al (8) showed that the initial inflammatory process of MS starts around small veins. Small (periventricular) lesions had a vein in their center (9). Kidd et al (10) showed a clear relationship between the site and characteristics of cortical lesions and five types of cortical veins. Large cortical lesions with extensive subcortical involvement and predominantly subcortical lesions seemed to arise as a result of involvement of the principal vein that passes through the cortex to the white matter, while smaller cortical lesions appeared to arise as a result of involvement of small veins or veins of the superficial regions of the cortex. The extensive venous vasculature of the cortex might explain the high prevalence of (enhancing) cortical lesions in MS, which in the study by Kidd et al (10) comprised 41 of 258 enhancing lesions. In our study, five (sub)cortical lesions were found, of which one showed strong contrast enhancement. This appeared to be the only lesion in which a central vein could not be depicted by venography. In contrast, in the same patient, another (less strongly enhancing) subcortical lesion had a central vein.
Because veins and venules are ubiquitous, they traverse most other types of disease processes. We incidentally used this technique in patients with hypoxic ischemic white matter lesions and found that, especially in extensive lesions, veins could be identified within such lesions. However, in contrast to MS lesions, these white matter lesions showed no relationship to the shape and location of the veins. Therefore, the mere presence of (small) veins in MS lesions may not be more than coincidence; only the correspondence of ovoid lesions along a vein is typical of MS.
Conclusion
High-resolution MR venography enables us to visualize, in vivo, the venous architecture in great detail in relation to MS lesions in the brain. The perivenous location of MS lesions can be clearly demonstrated and improves our understanding of their typical periventricular distribution and ovoid shape.
Acknowledgments
We thank Frank G. C. Hoogenraad and Mark B. M. Hofman from the Department of Clinical Physics & Informatics for technical support and Corline J. A. De Groot and Paul van der Valk from the Department of Pathology for reading the manuscript.
Footnotes
Presented in part at the annual meetings of the American Society of Neuroradiology, Philadelphia, May 1998; the European Committee for Treatment and Research in Multiple Sclerosis, Stockholm, September 1998; and the European Congress of Radiology, Vienna, March 1999.
Address reprint requests to I L. Tan, MD, Department of Radiology, University Hospital Vrije Universiteit, P.O. Box 7057, 1007 MB Amsterdam, the Netherlands.
References
- 1.Miller DH, Grossman RI, Reingold SC, McFarland HF. The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain 1998;121:3-24 [DOI] [PubMed] [Google Scholar]
- 2.Fog T. The topography of plaques in multiple sclerosis. Acta Neurol Scand 1965;15:1-161 [PubMed] [Google Scholar]
- 3.Raine C. The neuropathology of multiple sclerosis. In: Raine CS, McFarland HF, Tourtelotte WW, eds. Multiple Sclerosis: Clinical and Pathogenetic Basis. London: Chapman and Hall 1997 151-172
- 4.Horowitz AL, Kaplan RD, Grewe G, White RT, Salberg LM. The ovoid lesion: a new MR observation in patients with multiple sclerosis. AJNR Am J Neuroradiol 1989;10:303-305 [PMC free article] [PubMed] [Google Scholar]
- 5.Reichenbach JR, Venkatesan R, Schillinger DJ, Kido DK, Haacke EM. Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent. Radiology 1997;204:272-277 [DOI] [PubMed] [Google Scholar]
- 6.Poser CM, Paty DW, Scheimberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 1983;13:227-231 [DOI] [PubMed] [Google Scholar]
- 7.Reichenbach JR, Essig M, Haacke EM, et al. High-resolution venography of the brain using magnetic resonance imaging. MAGMA 1998;6:62-69 [DOI] [PubMed] [Google Scholar]
- 8.Adams CWM, Poston RN, Buk SJ. Pathology, histochemistry and immunocytochemistry of lesions in acute multiple sclerosis. J Neurol Sci 1989;92:291-306 [DOI] [PubMed] [Google Scholar]
- 9.Adams CWM. Vascular aspects of multiple sclerosis. In: A Colour Atlas of Multiple Sclerosis & Other Myelin Disorders. London: Wolfe Medical Publication 1989;184-187
- 10.Kidd D, Barkhof F, McConnel R, Algra PR, Allen IV, Revesz T. Cortical lesions in multiple sclerosis. Brain 1999;122:17-26 [DOI] [PubMed] [Google Scholar]