Abstract
Summary: We report the imaging findings in three symptomatic cases of partial aplasia of the posterior arch of the atlas with an isolated posterior remnant of the arch. These cases are instructive in illustrating the mechanism of cord impingement produced by the posterior arch remnant during extension of the cervical spine. Additionally, focal increased T2 signal was observed within the cord at the level of the anomaly in two of the patients.
Partial or complete absence of the posterior arch of the atlas is a rare although well-documented congenital anomaly (1–14). A subgroup of these patients have an isolated posterior tubercle along with a variable part of the posterior arch (5, 7, 8, 10–16). We describe the consequent cord compression by the isolated posterior arch remnant, with secondary neurologic findings in this subset of patients.
Case Reports
Case 1
A 32-year-old woman presented with episodic weakness and numbness of all four limbs after sustaining a minor head trauma. The symptoms were transient, lasting from a few minutes to an hour. The only positive finding on physical examination was the presence of brisk reflexes in both lower limbs. Plain radiographs of the cervical spine revealed bilateral defects in the posterior arch of the atlas with an isolated bony posterior arch remnant (Fig 1A and B). Radiographs taken in flexion and extension showed anterior displacement of the posterior arch remnant during extension (Fig 1A and B). MR imaging of the cervical spine (Fig 1C and D) showed partial aplasia of the posterior arch of the atlas and the presence of focal hyperintensity of the cord (Fig 1D) at a level just inferior to the lower limit of the posterior bony arch remnant. Cervical canal stenosis was evident from C2 through C5. No compression of the cord was noted at MR imaging, which had been carried out with the head in a neutral position. Excision of the isolated posterior bony fragment was advised, but the patient was subsequently lost to follow-up.
fig 1.
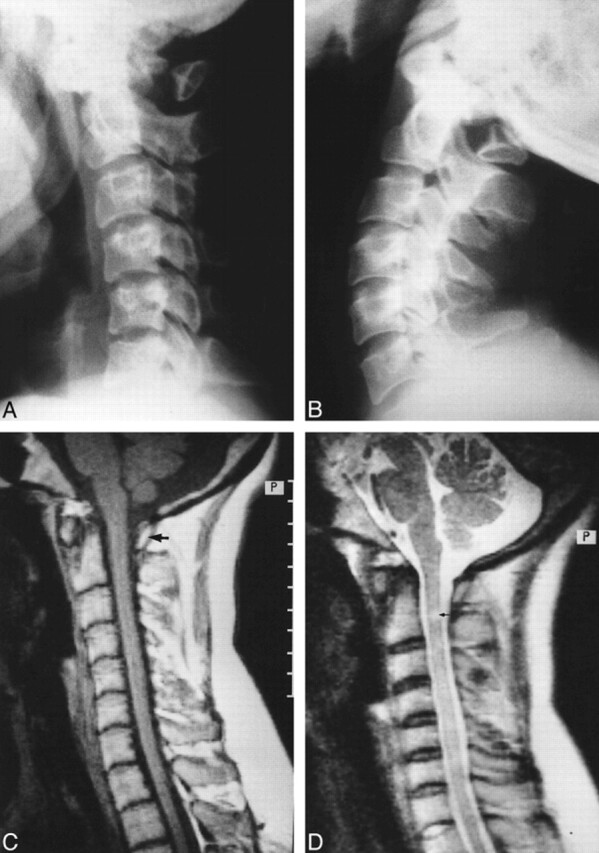
32-year-old woman with intermittent weakness and numbness of all four limbs.
A and B, Lateral radiographs of cervical spine taken in flexion (A) and extension (B) reveal partial aplasia of the posterior arch of the atlas with an isolated posterior bony fragment. Note the anterior displacement of the bony fragment during extension (B).
C, Midsagittal T1-weighted MR image through the cervical spine shows no compression of the cord by the posterior bony fragment (arrow).
D, Corresponding T2-weighted image shows a small, localized region of hyperintensity within the cord (arrow). Note that the signal alteration is seen slightly inferior to the anteroinferior margin of the isolated posterior arch remnant. A superimposed canal stenosis was noted from C2 through C5.
Case 2
A 35-year-old woman presented with neck pain of 6 months' duration and a tingling sensation and weakness in both upper limbs of 3 months' duration. Physical examination revealed minimal weakness in both upper extremities. All reflexes were normal, and plantar reflexes were downgoing. Plain radiographs of the cervical spine revealed aplasia of the posterior arch of the atlas with an intact posterior tubercle. No inward mobility of the posterior tubercle was observed during extension (Fig 2A and B). MR images of the cervical spine (Fig 2C and D) showed the presence of a small focal hyperintensity in the dorsal aspect of the cord just below the C1 level (Fig 2D). Focal canal stenosis was present at the C2–C3 disk level. No evidence of compression of the cord was seen on MR images at the level of the anomalous C1 arch. The patient did not undergo surgical intervention and, at the time of this writing, her symptoms had not progressed.
fig 2.
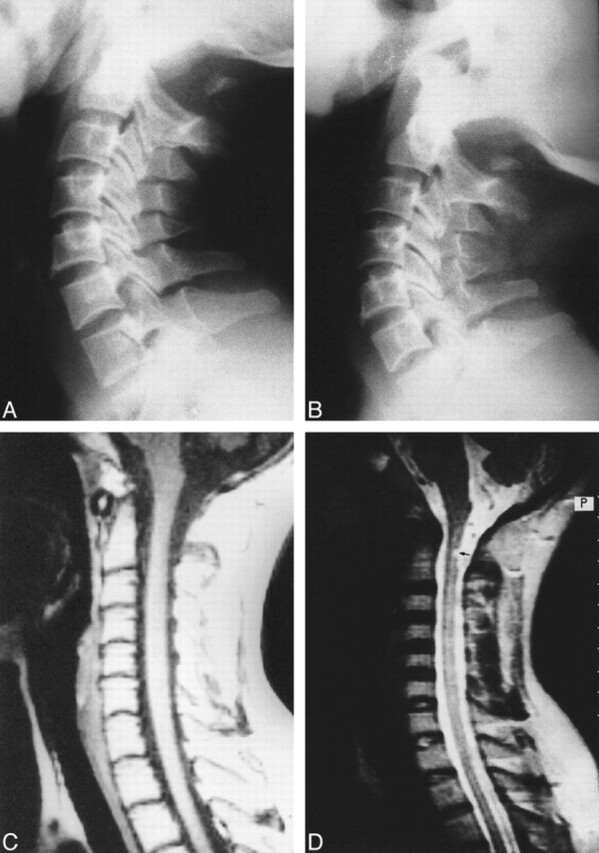
35-year-old woman with neck pain and paresthesias in both upper limbs.
A and B, In varying degrees of extension, plain radiographs show partial aplasia of the posterior arch of the atlas, with an isolated posterior tubercle. No obvious anterior displacement of the posterior tubercle is observed during extension.
C, Midsagittal T1-weighted MR image reveals no cord compression.
D, Corresponding T2-weighted image shows small, focal intramedullary hyperintensity (arrow) located just below the level of the posterior tubercle. In addition, there is focal canal stenosis at the C2–C3 disk level.
Case 3
A 30-year-old man presented with recurrent neck pain and stiffness for the past 5 years. He also reported intermittent tingling in both hands, lasting from a few hours to a few days. The patient had sustained a significant trauma in the form of a fall from a height of 50 feet prior to the onset of the symptoms. The findings on radiographs obtained after the trauma were reported as fractures in the posterior arch of the atlas. The patient was put in traction, and subsequently wore a cervical collar for several months, because subsequent radiographs showed no healing of the defects. The radiographs were reviewed at the time the patient presented to our hospital and were thought to reveal partial aplasia of the posterior arch. A subsequent CT scan showed the defects to be marginated by bone, with a smooth cortical outline. Radiographs taken during extension of the neck clearly showed anterior movement of the isolated posterior bony fragment (Fig 3A and B). MR images of the cervical spine failed to show any signal alteration within the cord. The presence of significant canal stenosis from C3 to C7 was also noted along with a superimposed disk bulge/protrusion at C5–C6 (Fig 3C and D). The patient was managed conservatively.
fig 3.
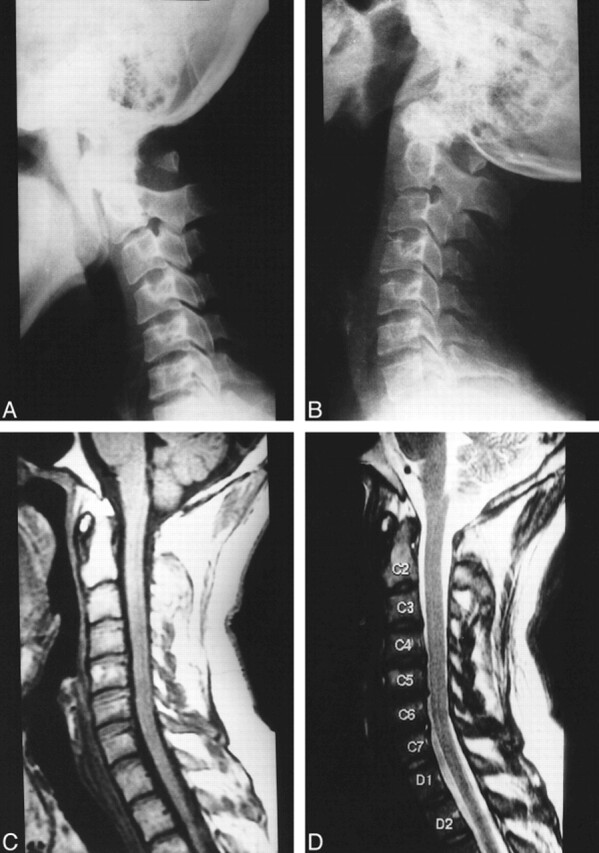
30-year-old man with neck pain and occasional upper limb paresthesias.
A and B, Flexion (A) and extension (B) plain radiographs of the cervical spine show partial aplasia of the posterior arch of the atlas with an isolated posterior arch remnant. The isolated posterior fragment moves anteriorly into the spinal canal during extension (B).
C and D, Midsagittal T1-weighted (C) and T2-weighted (D) MR images reveal no compression or signal alteration of the spinal cord. Significant canal stenosis from C3 to C7 is present along with a superimposed disk bulge/protrusion at C5–C6.
Discussion
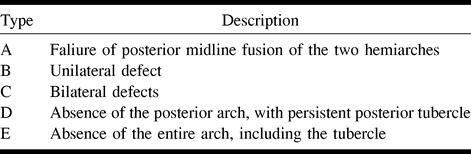
Congenital aplasia of the posterior arch of the atlas is a rare anomaly (1–14). Curriano et al (14) have divided this anomaly into five types (Table 1), depending on the extent of absence of the posterior arch and the presence or absence of the posterior tubercle. Our cases were of the type C (patients 1 and 3) and type D (patient 2) variety, and a common distinctive feature was the presence of an isolated dorsal remnant of the posterior arch.
Classification of congenital anomalies of the posterior arch of the atlas according to Curriano (14)
In general, the clinical presentation of reported cases has been variable. In their review of the literature, Curriano et al (14) found that almost one third of these patients have been asymptomatic with reference to the anomalous atlas. In some of the patients, transient (after trauma) or chronic neck or back pain was the presenting symptom (5, 8, 10, 12–14, 16). Overt neurologic symptoms are rare, and were seen in only 11 patients according to this review (14). Interestingly, 10 of the reported cases were similar to ours in that they had the isolated posterior arch remnant (14–17). This observation seems to suggest that the presence of an isolated posterior bony fragment somehow predisposes these patients to neurologic morbidity. The explanation lies in the hypothesis suggested by Richardson et al (15) to explain the symptoms of intermittent quadriplegia experienced by their patient with this anomaly. They suggested that the symptoms were secondary to compression of the cord by the inward mobility of the isolated posterior bony fragment during extension of the cervical spine (15).
The inward mobility of the posterior fragment during extension of the cervical spine was well demonstrated in two of our patients (Figs 1 and 3), and supports this hypothesis. It has been shown, both by autopsy studies and surgical findings, that the bony gap in the posterior arch of these patients is bridged by loose connective tissue rather than cartilage (2, 4, 6, 15). This has led to the belief that the anomaly is a result of defective development of the cartilage rather than a primary disturbance in ossification (9, 14). As a result, the posterior isolated bony fragment can move in dissociation from the anterior arch. Thus, impingement of the cord can result during extension, when the reduction in distance between the occiput and the spinous process of the axis leads to inward buckling of the attached ligaments, thereby displacing the bony fragment anteriorly, into the spinal canal.
One of our patients (case 2), with documented cord changes on MR images, did not show any anterior displacement of the isolated bony fragment during extension (Fig 2A and B). It is possible that in this patient, cord impingement occurred only in the presence of an unusual stress to the spine rather than during physiological movement of the spine. In addition, superimposed focal canal stenosis at the C2–C3 disk level may also have contributed to cord impingement. Although the patient did not have a history of significant trauma, it is possible that some trauma may have gone unnoticed, as similar cases have been reported in which presentation has followed trauma (14, 15, 17).
The role of MR imaging in these cases has not been discussed in detail in the literature. Apart from demonstrating bony abnormalities in our patients, MR imaging made a significant contribution by directly depicting the secondary changes within the spinal cord. These changes possibly represent focal myelomalacia, cord edema, or a “presyrinx” state. A unified concept to explain the syrinx formation and the changes in the cord that precede it have not yet been established. Recently, Fischbein et al (18) have postulated a plausible hypothesis to further the understanding of the pathophysiology of these cord abnormalities. Normal CSF diffusion along perivascular spaces within the spinal cord depends on the maintenance of proper systolic/diastolic pressure in the subarachnoid spaces. When this relationship is disturbed by increased pulsatile pressure (in our cases, cord impingement was caused by the inward movement of the C1 posterior arch remnant), it is possible that CSF directed into the extracellular space of the cord causes an abnormal amount of fluid to enter the central canal/cord parenchyma. If the situation persists long enough, the T2 signal abnormality may progress toward syringomyelia (18). As noted previously, in both our patients who had cord changes, the changes were observed at a level slightly below the level of the isolated bony fragment of the posterior arch (Figs 1D and 2D). This again indirectly supports the hypothesis that the cord impingement occurred during extension, when the cord is expected to move superiorly in relation to the posterior arch.
In this study, superimposed canal stenosis was found in all three patients, but cord changes were only present in two patients. In case 3, the lack of any MR signal alteration in the cord at the level of the anomalous C1 arch indicated the likelihood that the patient's symptoms were largely due to significant canal stenosis from C3 to C7, with a superimposed disk bulge/protrusion at C5–C6. From these three cases, it appears that symptoms are caused by multiple factors, such as positional inward mobility of the isolated bony fragment, minor or significant trauma, associated cervical osteoarthritis, or superimposed canal stenosis. None of our patients showed any direct evidence of cord compression at the level of the anomalous C1 arch. However, in all the patients, imaging was carried out with the neck in a neutral position. It is possible that cord compression could have been directly visualized had the MR study been performed with the neck extended. Depiction of this extension phenomenon during MR imaging (unfortunately, not performed by us) would have been more elegant, definitive, and novel.
Conclusion
On the basis of the findings in our three patients, we believe that the occurrence of an isolated posterior bony fragment should be viewed as a potential cause of significant neurologic morbidity rather than as an inconsequential normal variant.
Footnotes
Address reprint requests to Shailesh B. Gaikwad, MD, Neurosciences Centre, All India Institute of Medical Sciences, Ansari Nagar, New Delhi-110 029, India.
References
- 1.Geipel P. Zur Kenntnis der Spina bifida des Atlas. Forstschr Rontgenstr 1930;42:583-589 [Google Scholar]
- 2.Geipel P. Zur Kenntnis der Spaltbildung des Atlas und Epistropheus: Teil II. Forstschr Rontgenstr 1932;46:373-402 [Google Scholar]
- 3.Brown CE. Complete absence of the posterior arch of the atlas. Anat Rec 1941;81:499-503 [Google Scholar]
- 4.Geipel P. Zur Kenntnis der Spaltbildung des Atlas und Epistropheus: Teil III. Forstschr Rontgenstr 1935;52:533-570 [Google Scholar]
- 5.Lawrence WS, Anderson WD. Rare development abnormality of the atlas. Radiology 1937;28:55-57 [Google Scholar]
- 6.Geipel P. Zur Kenntnis der Spaltbildung des Atlas und Epistropheus: Teil IV. Zentralbl Allg Pathol 1955;94:19-84 [PubMed] [Google Scholar]
- 7.Von Torklus D, Gehle W. The Upper Cervical Spine. New York: Grune & Stratton; 1972
- 8.Logan WW, Stuart ID. Absent posterior arch of the atlas. AJR Am J Roentgenol 1973;118:431-434 [DOI] [PubMed] [Google Scholar]
- 9.Schultze P, Buurman R. Absence of the posterior arch of atlas. AJR Am J Roentgenol 1980;134:178-180 [DOI] [PubMed] [Google Scholar]
- 10.Dalinka MK, Rosenbaum AE, Van Houten F. Congenital absence of the posterior arch of atlas. Radiology 1972;103:581-583 [DOI] [PubMed] [Google Scholar]
- 11.Gehweiler JA, Daffner RH, Roberts L Jr. Malformation of the atlas simulating the Jefferson fracture. AJR Am J Roentgenol 1983;140:1083-1086 [DOI] [PubMed] [Google Scholar]
- 12.Raininko R, Virtama P. Hemiplasia of the posterior atlantic arch with a low lying posterior tubercle. Eur J Radiol 1984;4:4-5 [PubMed] [Google Scholar]
- 13.Dorne HL, Just N, Lander PH. CT recognition of anomalies of the posterior arch of the atlas vertebra: differentiation from fractures. AJNR Am J Neuroradiol 1986;7:176-177 [PMC free article] [PubMed] [Google Scholar]
- 14.Curriano G, Rollings N, Diehl JT. Congenital defects of the posterior arch of the atlas: a report of seven cases including an affected mother and son. AJNR Am J Neuroradiol 1994;15:249-254 [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson EG, Boone SC, Reid RL. Intermittent quadriplegia associated with a congenital anomaly of the posterior arch of atlas. J Bone Joint Surg 1975;57-A:853-854 [PubMed] [Google Scholar]
- 16.Spadaro A, Rotondo M, Conforti R, Muras I, Rinaldi F, Alberto R. Aplasia of the posterior arch of the atlas associated with isolated posterior tubercle. Acta Neurol Napoli 1987;9:19-25 [PubMed] [Google Scholar]
- 17.Holsten DR. Eine besondere form von defecbildung in hinteren atlasbogen. Forstschr Rontgenstr 1968;108:541-543 [PubMed] [Google Scholar]
- 18.Fischbein NJ, Dillon WP, Cobbs C, Weinstein PR. The “presyrinx” state: a reversible myelopathic condition that may precede syringomyelia. AJNR Am J Neuroradiol 1999;20:7-20 [PubMed] [Google Scholar]


