Abstract
Summary: We present the radiologic and pathologic findings in a boy who presented with diffuse leptomeningeal enhancement and whose clinical status deteriorated over the course of 5 years. During this period, MR images showed progression of the enhancement in the subarachnoid spaces, formation of intraaxial cysts, and hydrocephalus. Autopsy findings revealed diffuse oligodendroglioma throughout the leptomeninges of the brain and spine, with no definite intraaxial focus. The radiologic and pathologic features of diffuse leptomeningeal oligodendrogliomatosis are reviewed.
Diffuse leptomeningeal oligodendrogliomatosis is a rare disorder characterized by widespread invasion of the CSF-containing spaces by tumor without evidence of a primary intraparenchymal focus (1). Diagnosis of this entity is difficult, because imaging studies may show only progressive pial enhancement and hydrocephalus. We present the radiologic findings and their pathologic correlates of this rare disease.
Case Report
An 8-year-old boy presented with intermittent headaches. Over a 6-month period, his disease was characterized by fluctuating neurologic signs and symptoms, including papilledema, diplopia, ataxia, and upper extremity weakness. CSF analysis showed only elevated protein. Rheumatologic and infectious disease work-ups were unrevealing. Lysosomal enzyme and urine metabolic screens were negative. MR imaging of the brain showed thickened and abnormally enhancing subarachnoid spaces, particularly in the basal cisterns, sylvian fissures, and interhemispheric fissure (Fig 1). There was no hydrocephalus, and the presence of small cystic nonenhancing lesions in the left basal ganglia was noted. Our main differential diagnoses included tuberculosis, leptomeningeal tumor spread, histiocytosis, and idiopathic pachymeningitis. Within a year, increasing hydrocephalus, documented by CT, required ventriculoperitoneal shunt placement.
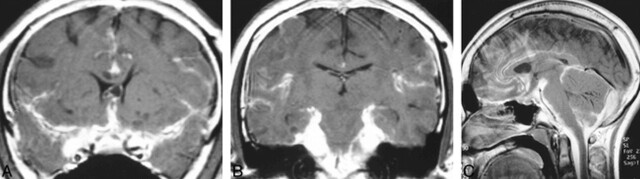
fig 1.
MR images obtained at initial presentation.
A, Coronal contrast-enhanced T1-weighted image shows diffuse enhancement of the subarachnoid space at the level of the suprasellar and sylvian fissure cisterns and along the interhemispheric fissure. There is no hydrocephalus, although there is questionable expansion of the cavum septum pellucidi. Small cystlike lesions are present in the left basal ganglia.
B, Coronal contrast-enhanced T1-weighted image posterior to A shows marked enhancement surrounding the brain stem.
C, Midsagittal contrast-enhanced T1-weighted image shows enhancement of the CSF-containing spaces, particularly at the base of the skull.
During the next 5 years, the patient was hospitalized on several occasions for progressive hydrocephalus, seizures, and intermittent encephalopathy. Biopsy samples of the meninges of the posterior fossa, obtained on two separate occasions, revealed a fibrocellular proliferation of unclear origin. MR imaging studies obtained 1 month before the patient's death showed intra- and extraaxial cystic lesions involving the basal ganglia and cerebellum as well as persistent enhancement of the subarachnoid spaces (Fig 2). The cysts had signal intensity similar to CSF on all sequences, and did not enhance. At this time, the diagnosis of gelatinous pseudocysts secondary to cryptococcosis was entertained, but the biopsy results did not support this hypothesis. A contrast-enhanced MR study of the cervical spine showed diffuse enhancement of the subarachnoid space and a small enhancing nodule within the spinal cord at the C6–C7 level (Fig 3A and B). The patient's final hospital admission was complicated by pneumonia and he died of sepsis and multiple organ failure. An autopsy revealed extensive necrotizing pneumonia caused by Legionella pneumophilia. Neuropathologic examination of the brain disclosed diffuse leptomeningeal oligodendrogliomatosis.
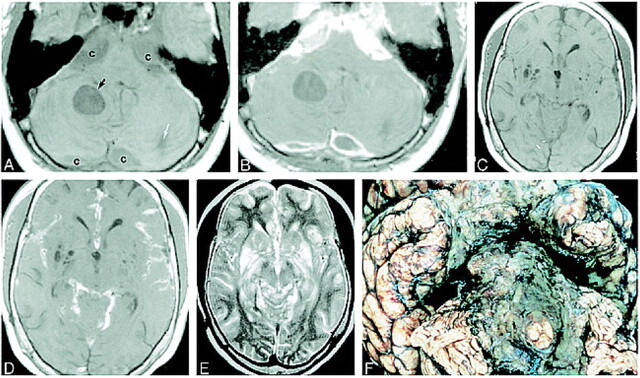
fig 2.
MR images obtained 1 month before death of patient and gross pathologic correlation.
A, Axial noncontrast T1-weighted image shows expansion of the cisterns (c), which are slightly inhomogeneous in appearance and of higher signal intensity than expected for normal CSF. There is a well-defined intraaxial cyst (black arrow) and a smaller one (white arrow) in the posterior left cerebellar hemisphere.
B, Corresponding contrast-enhanced T1-weighted image shows marked enhancement of the extraaxial abnormalities surrounding the brain stem. The cysts in the region of the cisterna magna show peripheral enhancement. The intraaxial cysts do not enhance.
C, Noncontrast axial T1-weighted image shows multiple cystlike lesions in the basal ganglia bilaterally.
D, Corresponding contrast-enhanced T1-weighted image shows enhancement of subarachnoid spaces in sylvian fissures and quadrigeminal plate and superior cerebellar cisterns. The lesions in the basal ganglia do not enhance. All lesions were hyperintense on T2-weighted images.
E, Axial T2-weighted image corresponding to C and D shows that the lesions in the cisterns, cortical sulci, and dilated perivascular spaces in the region of the basal ganglia are of high signal intensity.
F, Basal view of fixed brain shows thickened opaque leptomeninges encasing the brain stem and basal cisterns. These leptomeninges are diffusely infiltrated by tumor.
fig 3.
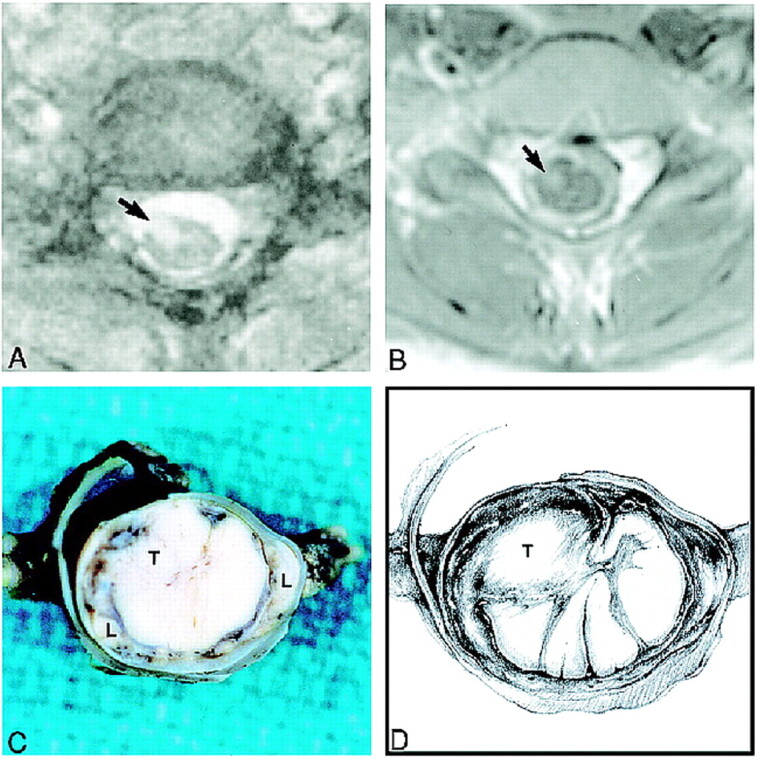
Spine MR images obtained during the last admission and gross pathologic correlation.
A, Axial T2-weighted image at the C6–C7 level shows an intramedullary spinal cord lesion (arrow). The subarachnoid spaces appear widened.
B, Corresponding contrast-enhanced T1-weighted image shows that the spinal cord lesion (arrow) enhances. The spinal cord appears hypointense and is surrounded by thick and enhancing subarachnoid space.
C, Fixed gross specimen shows a relatively well-circumscribed intramedullary tumor (T) within the cervical spinal cord surrounded by a dense cuff of leptomeninges (L).
D, Drawing depicts location of tumor nodule (T) and tumor infiltrating the leptomeninges.
Neuropathologic Studies
Postmortem evaluation showed diffusely thickened opacified leptomeninges covering the cerebrum, cerebellum, brain stem, and spinal cord (Fig 2F). The normal anatomic configuration of structures at the base of the brain was obscured by a dense mat of thickened leptomeninges, predominantly in the region of the optic chiasm and interpeduncular fossa. The basal cisterns showed marked leptomeningeal thickening and numerous loculated thin-walled cysts filled with clear serous fluid.
Gross sectioning of the cerebrum, cerebellum, and brain stem revealed dispersed small cystic lesions in the basal ganglia, cerebellar white matter, and medulla. Sections of spinal cord showed an intramedullary oval-shaped mass, 8 mm in diameter, which was relatively well circumscribed and located at the C6–C7 level (Fig 3C and D). Microscopy disclosed a generally monomorphous well-differentiated glial neoplasm that was pervasive throughout the subarachnoid space and that produced dense fibrosis in many areas. Focal superficial tumor invasion of the cerebral and cerebellar cortex was present (Fig 4A and B). Although regional variation in tumor-cell density was noted, both intracranial and spinal leptomeninges were involved by the neoplastic process. Foci of tumor-cell infiltrates were prominent within expanded perivascular spaces in sections of the basal ganglia and cerebellum, with rarefaction of surrounding neural tissue. Many large spherical eosinophilic granular bodies accompanied the neoplastic cells in the leptomeninges and neural tissue (Fig 4C). A single small neoplastic focus was seen in the spinal cord at the C6–C7 level (Fig 4D).
fig 4.
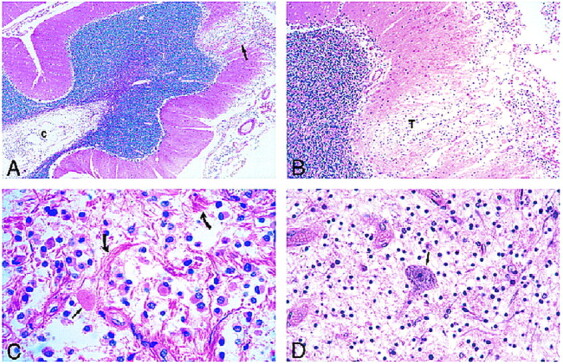
Photomicrographs of sections stained with hematoxylin-eosin.
A, Low-power view of the cerebellum shows leptomeningeal infiltrate and superficial cortical tumor invasion (arrow) with cystic rarefaction (c) of deep white matter by tumor.
B, Medium-power view of area of cortical tumor invasion shown in A reveals neoplastic cells (T) in a rarefied area of cortex.
C, The leptomeningeal infiltrate is composed of a monomorphous population of noncohesive cells with interspersed eosinophilic granular bodies (straight arrow) and marked fibrous proliferation (curved arrows).
D, View of spinal cord lesion shows an isolated anterior horn neuron (arrow) in a background of tumor cells.
The immunohistochemical profile of tumor cells included positive reactivity for Leu-7 and S-100 stains and negative reactivity for glial fibrillary acidic protein (GFAP), HMB-45, keratin, and leukocyte-common antigen stains (Fig 5). Leu-7 identifies a carbohydrate epitope associated with myelin and is present in the majority of oligodendrogliomas (2). HMB-45, keratin, and leukocyte-common antigen immunohistochemical stains were used to exclude the possibility of malignant melanoma, carcinoma, and lymphoid malignancies, respectively. Special stains for acid-fast bacilli and fungi were negative. Electron microscopic examination of sections obtained from grossly abnormal leptomeninges was noteworthy for a neoplastic population of cells characterized by round regular nuclei, inconspicuous nucleoli, and marginated chromatin with sparse cytoplasm (Fig 6). Rough endoplasmic reticulum was abundant. There was no evidence of cytoplasmic filaments, specialized intercellular junctions, or microorganisms. Spherical, eosinophilic, granular bodies, identified by light microscopy, were structurally consistent with lysosomal autophagic vacuoles. Infiltrating cells were morphologically and immunohistochemically consistent with oligodendrocytes. Nowhere was there evidence of a large intraparenchymal mass. The precise origin of the tumor was not demonstrable. The final pathologic diagnosis was disseminated oligodendrogliomatosis, primarily affecting intracranial and spinal leptomeninges, with multiple, small parenchymal tumor foci.
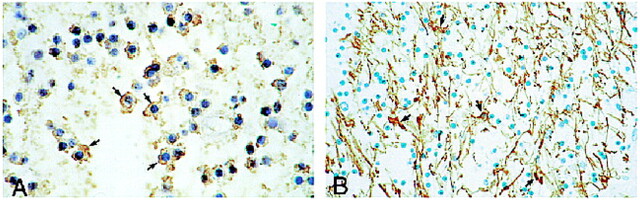
fig 5.
Immunocytochemistry.
A, High-power view shows positive immunoreactivity of neoplastic cells for Leu 7 (CD57). Note dark brown cytoplasmic immunostaining (arrows) of neoplastic oligodendrocytes.
B, Medium-power view shows a negative immunoreactivity of neoplastic cells for GFAP. Residual reactive astrocytes stain positively (arrows) whereas neoplastic cells are devoid of positive staining.
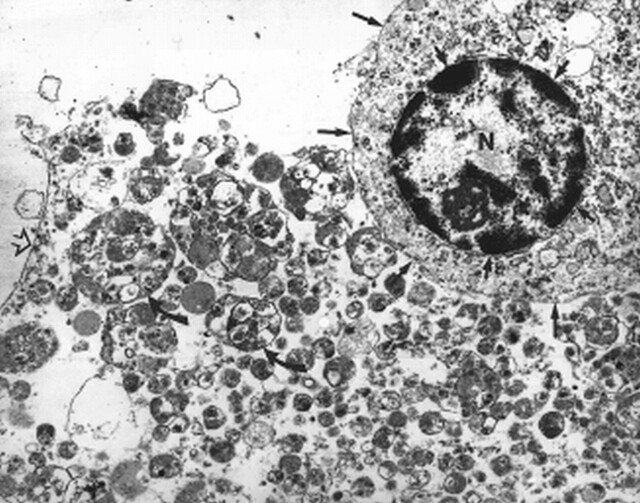
fig 6.
Electron microscopic view shows one tumor cell with a round regular nucleus (N), peripherally clumped chromatin (short arrows), and surrounding small amount of cytoplasm (long arrows point to cytoplasmic membrane). Adjacent to the tumor cell are numerous extracellular lysosomes (curved arrows) and a fragmented cell membrane (open arrow)
Discussion
Diffuse leptomeningeal gliomatosis is a well-recognized entity that results from invasion of the subarachnoid space or ventricular system by a primary intraparenchymal glioma; however, diffuse leptomeningeal oligodendrogliomatosis, in which a widely disseminated oligodendroglioma of the subarachnoid space overshadows a small or clinically undetected parenchymal focus, is rare. In our patient, a primary intraaxial focus was not detected, even at pathologic examination.
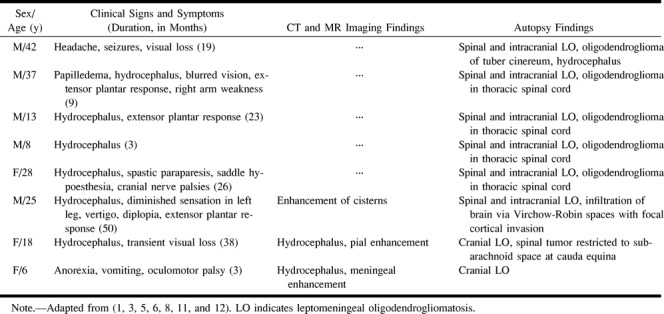
This case of diffuse leptomeningeal oligodendrogliomatosis is similar to previously reported cases (1, 3–9) (see Table 1), and all are different from primary diffuse leptomeningeal gliomatosis, which has been defined as a leptomeningeal-based glioma that has no “direct connection to the brain or spinal cord and . . . [is] . . . unaccompanied by a parenchymal neoplasm that could serve as a source of meningeal dissemination” (10). We found only two cases of primary diffuse leptomeningeal oligodendrogliomatosis reported in the literature (8, 9). It has been postulated that gliomas that primarily involve the subarachnoid space arise from leptomeningeal heterotopias (4). These congenital anomalies consist of small islands and nests of glial tissue within the subarachnoid space and occur in approximately 1% of healthy individuals and 25% of patients with other congenital brain malformations (11).
Disseminated leptomeningeal oligodendrogliomatosis as reported in the literature
Antemortem diagnosis of diffuse leptomeningeal oligodendrogliomatosis is difficult owing to nonspecific neurologic signs and symptoms, a frequently fluctuating clinical course, and negative cytologic examination of CSF for neoplastic cells. Signs of progressively increasing intracranial pressure and meningeal abnormalities by imaging studies often warrant a biopsy. As in our patient, the diagnostic yield may be low because of regional variations in the density of neoplastic cells and the focally prominent fibrosis within the leptomeninges.
A salient neuroradiologic finding in our case was multiple intraparenchymal cysts in the basal ganglia, cerebellum, and medulla. Postmortem examination revealed that these lesions were due to tumor-cell infiltration and expansion of the perivascular spaces, with focal rarefaction of surrounding neural tissue. Electron microscopic evaluation showed a neoplastic population of cells morphologically consistent with oligodendrocytes characterized by round regular nuclei, sparse cytoplasm, and prominent rough endoplasmic reticulum. In general, oligodendroglial tumors lack bundles of cytoplasmic glial filaments, which are a characteristic finding in astrocytic lesions. Specialized intercellular junctions are an ultrastructural feature of epithelial cells and are often present in carcinomas. In our patient, tumor cells showed no evidence of intercellular junctions. An unusual finding at light microscopy examination were abundant, plump, eosinophilic granular bodies accompanying the tumor cells. Upon ultrastructural examination, these structures proved to be cell forms, delimited by cell membranes and containing numerous autophagic lysosomes. Autophagic lysosomes provide sites for intracellular digestion and turnover of cellular components. Cytoplasmic digestion by autophagic lysosomes is enhanced during physiological remodeling of cells (ie, differentiation and atrophy). It has been postulated that neoplastic cells, like normal cells, may show increased autophagic activity as a result of altered environmental conditions, such as hypoxia (12). Structures similar to the eosinophilic granular bodies noted in this case have been reported in conventional oligodendrogliomas and in leptomeningeal oligodendrogliomatosis (7, 12).
Footnotes
Address reprint requests to Mauricio Castillo, MD, Department of Radiology, Campus Box 7510, University of North Carolina School of Medicine, Chapel Hill, NC 27599.
References
- 1.Beck DJK, Russell DS. Oligodendrogliomatosis of the cerebrospinal fluid pathway. Brain 1942;65:352-372 [Google Scholar]
- 2.Perentes E, Rubinstein LJ. Recent applications of immunoperoxidase histochemistry in human neuro-oncology. Arch Pathol Lab Med 1989;111:796-812 [PubMed] [Google Scholar]
- 3.Russell DS. Observations on the pathology of hydrocephalus. In: Medical Research Council, Special Report, Series No. 265. London: His Majesty's Stationary Office; 1949 112-113
- 4.Michel D, Lemercier G, Beau G, Tommasi M, Schott B. Gliomatose méningée et ventriculaire diffuse secondaire à un oligodendrogliome intramédullaire: a propos d'une observation. Lyon Med 1975;234:37-41 [Google Scholar]
- 5.Toso V. Diffusioni metastiche alle leptomeningi. Acta Neurol Napoli 1967;22:366-376 [PubMed] [Google Scholar]
- 6.Wöber G, Jellinger K. Intramedulläres oligodendroliom mit meningozerebraler aussaat. Acta Neurochir 1976;35:261-269 [DOI] [PubMed] [Google Scholar]
- 7.Rogers L, Estes M, Rosenbloom SA, Harrold L. Primary leptomeningeal oligodendroglioma: case report. Neurosurgery 1995;36:166-169 [DOI] [PubMed] [Google Scholar]
- 8.Chen R, Chir B, MacDonald DR, Ramsay DA. Primary diffuse leptomeningeal oligodendroglioma. J Neurosurg 1995;83:724-728 [DOI] [PubMed] [Google Scholar]
- 9.Ng HK, Poon WS. Diffuse leptomeningeal gliomatosis with oligodendroglioma. Pathology 1999;31:59-63 [DOI] [PubMed] [Google Scholar]
- 10.Burger P, Scheithauer BW, Vogel FS. Surgical Pathology of the Nervous System and Its Coverings.. 3rd ed. New York: Churchill Livingstone; 1991:117
- 11.Cooper IS, Kernohan JW. Heterotopic glial nests in the subarachnoid space: histopathologic characteristics, mode of origin and relation to meningeal gliomas. J Neuropathol Exp Neurol 1951;10:16-29 [DOI] [PubMed] [Google Scholar]
- 12.Takei Y, Mirra S, Miles L. Eosinophilic granular cells in oligodendrogliomas. Cancer 1976;38:1968-1976 [DOI] [PubMed] [Google Scholar]