Abstract
BACKGROUND AND PURPOSE: Although various particulate materials have been developed as embolization agents, their biocompatibility remains unclear. We used an animal model to examine the possibility of using FDA-approved microcrystalline cellulose spheres (CELPHERE) as solid embolic material for the permanent occlusion of blood vessels.
METHODS: Angiographic and histologic studies in 12 canine renal arterial systems were conducted to evaluate the performance of CELPHERE beads at 1 hour, and at 4 and 12 weeks after embolization.
RESULTS: The CELPHERE beads traveled to vessels with diameters approximating their own. Larger vessels were occluded by aggregations of beads. There was no disruption of vessel walls and no evidence of perivascular hemorrhage or inflammatory changes.
CONCLUSION: Because CELPHERE beads are easy to handle, highly biocompatible, and have few adverse effects, they are suitable for intravascular applications.
When embolization is deemed appropriate for the treatment of vascular malformations and neoplasms, the embolic agent must be selected with utmost care. Various particulate materials have been developed and tested in animal models or used in clinical settings. Some of these materials have proved unsatisfactory: some because they were found to be absorbable, others required tedious preparative steps, were not suspended equally, produced high levels of friction, or resulted in incomplete occlusion. The ideal permanent embolic material must exhibit the following characteristics: it must be nonabsorbable, spherical, and smooth-surfaced; it must be able to calibrate its size accurately; and its specific gravity must be close to that of whole blood. For superselective and permanent vessel occlusion, these conditions must be met. Experience with some spherical materials meeting most of these criteria has been reported (1–8), and the development of biomaterials has entered a new phase. Although the newest materials tend not to produce severe tissue reactions after permanent occlusion, none have yet been approved by the U.S. Food and Drug Administration (FDA) and their long-term safety has not yet been established.
Although we previously developed an ideal particulate embolic material, the cellulose porous bead (CPB) (9–11), the FDA has not yet approved it. Therefore, we continue our quest for a new embolic agent composed of material that could gain FDA approval.
We tested a particulate material, the microcrystalline cellulose sphere (CELPHERE) bead, that meets all of the criteria listed above and, in addition, offers advantages related to both morphologic and biological compatibility with vessels. We carefully examined the properties and characteristics of CELPHERE beads, and studied embolization techniques in a dog model as a preliminary step toward eventual clinical use in arterial embolization.
Methods
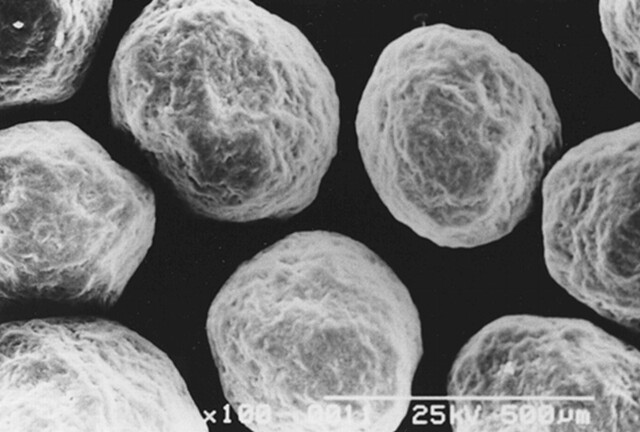
Celphere embolization beads (Asahi-Kasei Co. Ltd., Tokyo, Japan) are newly developed microspheres that are manufactured commercially as structural elements of tablets. They are made of 100% Avicel microcrystalline cellulose—a purified, partially depolymerized alpha cellulose derived from purified specialty grades of wood pulp. Microcrystalline cellulose is a primary excipient in pharmaceutical oral dosage formulations because of its very low reactivity to active ingredients. It is white, tasteless, odorless, virtually free of organic and inorganic contaminants, and recognized as safe by experts. It is FDA-approved and is listed in the Drug Master File as “Microcrystalline Cellulose Spheres, CELPHERE”. Microcrystalline cellulose spheres are insoluble in water and most organic solvents; they are resistant to degradation in water and are water-absorbent. The insolubility and water-absorbent properties of the beads result in reduced particle agglomeration. They are fundamentally globoid in shape and come in different diameters (150, 200, 300, 500 μm). They are of uniform and accurately calibrated size; the mean error of their diameter is within 30 μm. Their surface contour is smooth under an electron microscope (Fig 1).
fig 1.
Scanning electron micrograph of 200-μm -diameter CELPHERE beads. They are spherical and of accurately calibrated size. The bar represents 500 μm
CELPHERE beads of two different sizes (150 and 200 μm in diameter) were suspended in iodinated contrast material in stoppered glass bottles at about 1000 CELPHERE beads per mL and sterilized for 20 minutes in a steam autoclave at 121° C at 11 Pa. The number of beads in the suspension was obtained by counting aliquots with a Coulter multisizer 2 (Coulter Electronics Limited, Beds, England). For each batch, the quality of sterilization was bacteriologically confirmed.
In the in vivo experiments, we used 12 adult mongrel dogs weighing about 15 kg. They were premedicated intramuscularly with 0.01 mg atropine sulfate and 10 mg ketamine chloride per kg. Prior to intratracheal intubation, they were anesthetized with IV injection of 10 mg thiopental sodium per kg. Additional thiopental sodium (5mg/kg) was IV injected as needed. Muscle relaxation was produced with suxamethonium chloride. The experiments were performed under controlled mechanical ventilation. During the experiment, the arterial carbon dioxide pressure was maintained between 35 and 40 mm Hg; the partial pressure of arterial oxygen was kept at 100 mm Hg or higher. The right femoral artery in the groin was punctured, a 4F introducer sheath was placed, and 2000 U heparin was injected. At the end of the procedure, protamine sulfate was injected to reverse the effect of heparin.
Renal preembolization angiograms were obtained using a 4F polyethylene catheter with the tip of the catheter positioned in the orifice of the artery. Under fluoroscopic control, a unilateral embolization of the renal artery was performed slowly until the flow was arrested (usually after 6 mL). Suspended in the embolization volumes were 1000 CELPHERE beads/mL. Selective renal angiograms were obtained immediately and at 1 hour, and again at 4 and 12 weeks after embolization. All dogs, except those sacrificed 1 hour after embolization (n=4), were allowed to recover from anesthesia. The latter were sacrificed 4 weeks (n=4) and 12 weeks (n=4) after the procedure. Two animals in each group underwent embolization with CELPHERE beads measuring 150 and 200 μm in diameter, respectively. The kidneys were removed en bloc, fixed in 10% neutral buffered formalin, cut into 3-micron sections, and stained with hematoxylin & eosin, Giemsa, and Elastica van Gieson.
In all experiments, we followed the Animal Care Guidelines set forth by the Animal Experimentation Ethical Committee of our institution.
Results
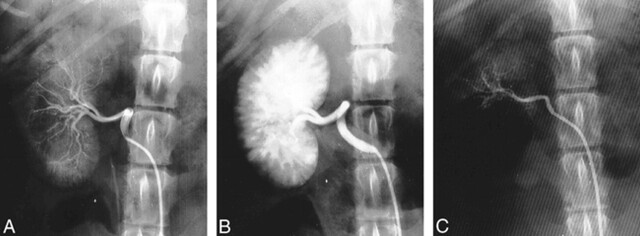
None of the dogs manifested any outward signs of abnormality during the pre- and postembolization observation period. Renal angiograms, obtained before embolization, showed typical canine arterial vasculature. CELPHERE produced a smooth reduction in blood flow; severe stasis occurred in the immediate phases after embolization of the main renal artery. Renal angiograms obtained 1 hour and 4 and 12 weeks after embolization revealed complete occlusion of the renal artery (Fig 2).
fig 2.
A, Preembolization angiogram shows the typical canine renal arterial system.
B, Angiogram obtained 1 hour after embolization shows complete occlusion of the main renal artery and the radiodensity of the CELPHERE cast.
C, Angiogram obtained 12 weeks after embolization shows no evidence of recanalization of the embolized main renal artery.
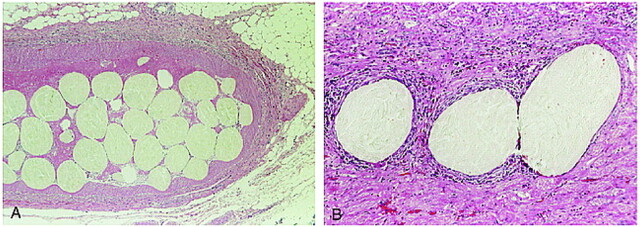
On examination of the gross kidney specimens, thrombosis of the main renal artery was noted. During the 12 weeks after embolization, a progressive, marked decrease in renal size occurred. Microscopically, CELPHERE beads measuring 200 μm in diameter were distributed throughout the intramedullary arteries; beads measuring 150 μm in diameter were observed in the arteries on the border of the cortex and medulla. The CELPHERE beads traveled to vessels with diameters approximating their own. Larger vessels were occluded by dense aggregates of many beads; there were no interstices (Fig 3A). There was no damage to vessel walls and no evidence of perivascular hemorrhage. Sections obtained 1 hour after embolization showed fresh thrombus formation. Sections prepared 4 and 12 weeks after embolization showed permanent occlusion of the vessels with infarction. There were no inflammatory changes of the vessel walls. The particles were not resorbed, nor did their appearance change during the 12-week observation period (Fig. 3B).
fig 3.
Section obtained 12 weeks after embolization. Large vessels were packed with aggregated CELPHERE beads. The beads traveled to vessels approximately 200 μm in diameter without stretching the vessel wall or rupturing the internal elastic lamina. There is no evidence of perivascular hemorrhage or inflammatory change in the peripheral area. (Hematoxylin & eosin stain: A, original magnification ×40; B, original magnification ×100)
Discussion
We have been testing embolic materials with the goal of detecting some that are superior with respect to ease and safety of use, biocompatibility, and permanence of embolization. We previously developed the solid embolic agent, CPB, which is still undergoing clinical trial. These beads are exceptionally uniform in size, and have a specific gravity similar to blood and net-positive charge. Their long-term safety, however, has not been established. CELPHERE beads are microspheres that are produced commercially; the FDA has approved their use in tablet form for humans.
Embolization with CELPHERE did not result in inflammation to surrounding tissues even 12 weeks after embolization, nor was there any apparent damage to vessel walls, angionecrosis, or focal hemorrhage. Germano et al (12) reported that polyvinyl alcohol (PVA) particles induced a rapid foreign-body response and inflammation in all embolized vessels. This could result in arterial rupture or aneurysm formation. Angionecrosis, focal hemorrhage, and severe vasogenic edema occurred in rare cases. Although foreign-body giant cells are a nonspecific reaction to many foreign materials and do not by themselves indicate toxicity, the presence of localized angionecrosis and focal hemorrhages strongly suggested direct toxicity of the particles (12–15).
The CELPHERE beads used in our study were spherical in shape, their surface was smooth, and it was possible to calibrate their size accurately. The beads migrated distally without clumping, thereby producing a more complete and permanent occlusion. In fact, we encountered no recanalization as late as 12 weeks after embolization.
Because CELPHERE beads come in different sizes (150, 200, 300, 500 μm in diameter with a mean error of 30 μm), the optimal size can be selected on a case-by-case basis. Their tendency to travel to vessels with diameters approximating their own resulted in a high embolic effect. The propensity of some embolic materials (eg, PVA and Sephadex) to become enlarged after introduction into the bloodstream resulted in stretching and, in some cases, rupture of the internal elastic lamina and vessel wall (16). Our experiments showed that the CELPHERE beads retained their size even as late as 12 weeks after injection, and that they produced no injury to the vessel walls.
The apparent density of CELPHERE beads was 0.87–0.97 g/cm3, and the specific gravity was close to that of whole blood. Although CELPHERE beads of 150 and 200 μm diameter traveled easily through the length of the microcatheter (Tracker 18, Guerbet Biomedical, Target Therapeutics), there was slight resistance at the microcatheter connector. To optimize the characteristics of CELPHERE beads for permanent embolization, a desirable improvement would be to reduce their gravity to allow for prolonged suspension.
Conclusion
Our animal study showed that CELPHERE beads satisfied most of the requirements of an ideal permanent embolic material. Further slight improvements will make it possible to perform clinical trials with this embolic material.
Footnotes
Address reprint requests to Yutaka Kai, M.D., Department of Neurosurgery, Kumamoto University Medical School, 1-1-1 Honjo, Kumamoto 860-8556, Japan.
References
- 1.Beaujeux R, Laurent A, Wassef M, et al. Trisacryl gelatin microspheres for therapeutic embolization. II: Preliminary clinical evaluation in tumors and arteriovenous malformations. AJNR Am J Neuroradiol 1996;17:541-548 [PMC free article] [PubMed] [Google Scholar]
- 2.Dion JE, Rankin RN, Vinuela F, Fox AJ, Wallace AC, Mervart M. Dextran microsphere embolization: Experimental and clinical experience with radiologic-pathologic correlation. Work in progress. Radiology 1986;160:717-721 [DOI] [PubMed] [Google Scholar]
- 3.Flandroy P, Grandfils C, Collignon J, et al. ( D,L) polylactide microspheres as embolic agent. A preliminary study. Neuroradiology 1990;32:311-315 [DOI] [PubMed] [Google Scholar]
- 4.Gu WZ, Link DP, Tesluk H, Blashka K. Experimental renal embolization: Preliminary results with polyacrylonitrile-based multiblock copolymers. J Vasc Interv Radiol 1992;3:119-125 [DOI] [PubMed] [Google Scholar]
- 5.Horak D, Svec F, Kalal J, et al. Hydrogels in endovascular embolization. II. Clinical use of spherical particles. Biomaterials 1986;7:467-470 [DOI] [PubMed] [Google Scholar]
- 6.Horak D, Svec F, Kalal J, et al. Hydrogels in endovascular embolization. I. Spherical particles of poly (2-hydroxyethyl methacrylate) and their medico-biological properties. Biomaterials 1986;7:188-192 [DOI] [PubMed] [Google Scholar]
- 7.Laurent A, Beaujeux R, Wassef M, Rufenacht D, Boschetti E, Merland JJ. Trisacryl gelatin microspheres for therapeutic embolization, I: development and in vitro evaluation. AJNR Am J Neuroradiol 1996;17:533-540 [PMC free article] [PubMed] [Google Scholar]
- 8.Turjman F, Massoud TF, Vinters HV, et al. Collagen microbeads: experimental evaluation of an embolic agent in the rete mirabile of the swine. AJNR Am J Neuroradiol 1995;16:1031-1036 [PMC free article] [PubMed] [Google Scholar]
- 9.Hamada J, Ushio Y, Kazekawa K, Tsukahara T, Hashimoto N, Iwata H. Embolization with cellulose porous beads, I: An experimental study. AJNR Am J Neuroradiol 1996;17:1895-1899 [PMC free article] [PubMed] [Google Scholar]
- 10.Hamada J, Kai Y, Nagahiro S, Hashimoto N, Iwata H, Ushio Y. Embolization with cellulose porous beads, II: Clinical trial. AJNR Am J Neuroradiol 1996;17:1901-1906 [PMC free article] [PubMed] [Google Scholar]
- 11.Connors JJ III, Wojak JC. Interventional Neuroradiology: Strategies and Practical Techniques. Philadelphia: Lippencott 1999 28
- 12.Germano IM, Davis RL, Wilson CB, Hieshima GB. Histopathological follow-up study of 66 cerebral arteriovenous malformations after therapeutic embolization with polyvinyl alcohol. J Neurosurg 1992;76:607-614 [DOI] [PubMed] [Google Scholar]
- 13.Davidson GS, Terbrugge KG. Histologic long-term follow-up after embolization with polyvinyl alcohol particles. AJNR Am J Neuroradiol 1995;16:843-846 [PMC free article] [PubMed] [Google Scholar]
- 14.Quisling RG, Mickle JP, Ballinger WB, Carver CC, Kaplan B. Histopathologic analysis of intraarterial polyvinyl alcohol microemboli in rat cerebral cortex. AJNR Am J Neuroradiol 1984;5:101-104 [PMC free article] [PubMed] [Google Scholar]
- 15.Wakhloo AK, Juengling FD, Van Velthoven V, Schumacher M, Hennig J, Schwechheimer K. Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: assessment of two embolization techniques. AJNR Am J Neuroradiol 1993;14:571-582 [PMC free article] [PubMed] [Google Scholar]
- 16.Wright KC, Anderson JH, Gianturco C, Wallace S, Chuang VP. Partial splenic embolization using polyvinyl alcohol foam, dextran, polystyrene, or silicone. An experimental study in dogs. Radiology 1982;142:351-354 [DOI] [PubMed] [Google Scholar]