Abstract
BACKGROUND AND PURPOSE: Cerebral arteriovenous malformations (AVMs) are occasionally associated with hereditary hemorrhagic telangiectasia (HHT), which is characterized by the presence of multiple mucocutaneous telangiectasia, epistaxis, and familial inheritance. We analyzed the angiographic and clinical characteristics of patients with cerebral AVMs related to HHT.
METHODS: Among 638 patients with cerebral AVMs, we identified 14 patients with HHT. The AVMs were classified as those with nidi of 1 cm or less (micro AVMs), those with nidi between 1 and 3 cm (small AVMs), and those of the fistulous type (arteriovenous fistulas [AVFs]).
RESULTS: A total of 28 AVMs were found; seven of 14 patients had multiple AVMs. The 28 AVMs were categorized as 12 micro AVMs, eight small AVMs, and eight AVFs. All except one micro AVM were asymptomatic, whereas all small AVMs were symptomatic. Three of eight AVFs were asymptomatic. All 28 AVMs were located on the cortex. All micro AVMs and AVFs had single feeders and single draining veins, whereas the small AVMs had multiple feeders in all lesions and single draining veins in six of eight lesions.
CONCLUSION: Multiple, cortical, micro AVMs or AVFs harboring single feeding arteries and single draining veins should raise clinical suspicion of HHT-related AVMs.
Hereditary hemorrhagic telangiectasia (HHT), also called Rendu-Osler-Weber syndrome, is an autosomal dominant vascular disorder characterized by mucocutaneous telangiectases and visceral arteriovenous malformations (AVMs). The mucocutaneous telangiectases frequently lead to chronic epistaxis and gastrointestinal bleeding. AVMs occur most frequently in the lung, brain, and liver. The prevalence of HHT is estimated to be 1:10 000 (1). Two different genes that can be mutated in cases of HHT have been discovered: the endoglin gene on chromosome 9 and the activin receptor-like kinase gene on chromosome 12 (2−4). Reports suggest that 2% of cerebral AVMs are associated with HHT (5) and that 5% to 10% of patients with HHT have cerebral AVMs (6, 7). The clinical and angiographic characteristics of cerebral AVMs associated with HHT, however, have not been well described. We reviewed the HHT-related cerebral AVMs from our brain vascular malformation experience, focusing on morphologic characteristics revealed by angiography.
Methods
We retrospectively reviewed the records of 14 patients with cerebral AVMs and HHT from 638 consecutive cases of cerebral AVMs managed at our institution from October 1982 to April 1998. Two patients had a known diagnosis of HHT. The remaining 12 patients were subsequently diagnosed with HHT based on an HHT screening protocol (questionnaire and clinical assessment). Screening is ongoing; therefore, the prevalence of HHT is very likely an underestimate. To establish a diagnosis of HHT, a patient must meet at least two of the following criteria: recurrent epistaxis, typical mucocutaneous telangiectasia, positive family history of HHT with a first-degree relative affected, and a typical AVM (8).
All patients underwent complete cerebral angiography. Some patients underwent superselective angiography that was achieved using a microcatheter. Ten of the 14 patients underwent 1.5-T MR imaging of the brain without the administration of contrast material, and the remaining four patients underwent CT of the brain. Angiography was used to evaluate the angioarchitecture of the lesions, which includes number of AVMs, size and location of AVMs, origin and number of feeding arteries, location of draining veins, and occurrence of venous pouches. MR imaging or CT or both were used to evaluate size and location of AVMs. Based on the imaging studies, these lesions were divided into arteriovenous fistulas (AVFs), micro AVMs with nidi of 1 cm or less, and small AVMs with nidi between 1 cm and 3 cm (Figs 1 and 2). In our analysis, cortical lesions included AVMs that involved the cortex irrespective of their extension of the ventricle (9).
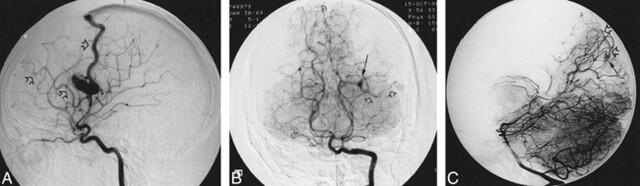
fig 1.
Angiograms of a 21-year-old man who presented with a grand mal seizure, a history of epistaxis, lower-lip telangiectasia, and a family history of HHT.
A, Lateral-view angiogram of the right internal carotid artery shows the small AVM involving right parietal lobe cortex filled by the two branches of the right middle cerebral artery with two draining veins (open arrows).
B, Anteroposterior-view angiogram of the vertebral artery shows a second AVM (micro AVM) (arrow), involving the left occipital cortex, fed by a branch of the posterior cerebral artery and draining into a single vein (open arrows).
C, Lateral-view angiogram shows another micro AVM (arrow) involving the right occipital lobe cortex supplied by a parieto-occipital artery with a single draining vein (open arrows).
Results
Clinical Presentation
Patient age ranged from 7 to 59 years (mean, 27.0 years). There were nine female and five male patients. The initial symptoms were seizure in six patients, cerebral hemorrhage in three, headache in three, and two patients were asymptomatic. The first asymptomatic patient was known to have pulmonary AVMs and therefore underwent MR imaging to screen for cerebral AVMs. In the second asymptomatic patient, the cerebral AVM was noted incidentally during angiography of the carotid artery, while the patient was being investigated for a tongue AVM. Other HHT manifestations in this cohort were frequent epistaxis in 10 patients, mucocutaneous telangiectasis in nine, gastrointestinal bleeding in four, pulmonary AVMs in three, pancreas AVM in one, tongue AVM in one, and liver AVM in one. The follow-up period ranged from 7 months to 12 years (mean, 4 years 11 months). During this follow-up period, eight of 28 AVMs that had not been treated remained asymptomatic.
Angiographic Analysis
Twenty-eight cerebral AVMs were found in 14 patients. Multiple AVMs were noted in seven (50%) of the 14, ranging from two to four AVMs per patient.
Each of 20 of the cerebral AVMs had a nidus, whereas eight were of the fistulous type. Twelve AVMs measured 1 cm or less, and eight measured between 1 cm and 3 cm. Therefore, of the 28 AVMs, there were 12 (42.9%) micro AVMs, eight (28.6%) small AVMs, and eight (28.6%) AVFs.
Of the 12 micro AVMs, six occurred in children (15 years or younger). Seven of eight small AVMs occurred in adults. All AVFs occurred in children.
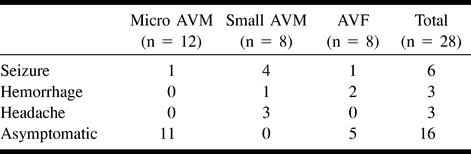
The relationship of symptoms at presentation to AVM angioarchitecture was assessed (Table 1). The micro AVMs were asymptomatic in 11 (91.7%) patients and presented with seizures in one (8.3%). No micro AVM caused intracranial hemorrhage in our study. These 11 asymptomatic AVMs were diagnosed during cerebral angiography, which was performed to investigate other symptomatic cerebral AVMs or other HHT symptoms. The small AVMs presented with seizures in four (50.0%) patients, intracranial hemorrhage in one (12.5%), and headache in three (37.5%). The AVFs presented with seizures in one (12.5%) patient and with intracranial hemorrhage in two (25.0%) and were asymptomatic in five (62.5%).
TABLE 1:
Relationship between initial presentation and cerebral AVM type in HHT (n = 28)
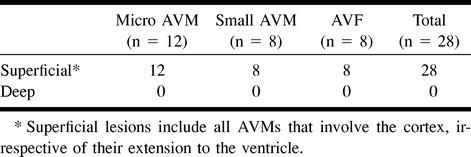
All three types of AVMs were situated on the cerebral cortex (Table 2). No AVMs were seen in the basal ganglia or brain stem. Twenty-five (89.3%) AVMs were supratentorial and three (10.7%) were infratentorial.
TABLE 2:
Cerebral AVM location in HHT (n = 28)
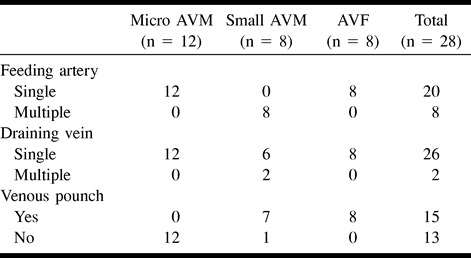
The characteristics of the feeding arteries and draining veins are described in Table 3. On the arterial side, it was noted that all micro AVMs had single feeding arteries, whereas all small AVMs had multiple feeders. All AVF lesions had single feeders and single fistulas. On the venous side, it was noted that all AVMs had single draining veins, with the exception of two small AVM lesions. All small AVMs except one and all AVF lesions had venous pouches, whereas no micro AVMs had venous pouches. Superficial draining veins were found in 22 (78.6%) of 28 AVMs.
TABLE 3:
Angioarchitecture of cerebral AVMs in HHT (n = 28)
Discussion
The most striking finding regarding cerebral AVMs associated with HHT is their multiplicity. In our study, each of seven (50%) of 14 patients were found to have two or more AVMs. A limited number of cases have been reported in the literature of HHT-related cerebral AVMs (5, 10−23). A meta-analysis of these reports shows multiple cerebral AVMs in 25 (50.0%) of 50 patients. In sporadic AVMs, our data indicated multifocal AVMs in six (1.0%) of 624 patients, which is in keeping with the 0.7 to 3% prevalence reported in the literature (23, 24). The rate of multiplicity of AVMs in patients with HHT clearly overwhelms such occurrence in patients with sporadic AVMs.
Another specific finding associated with cerebral AVMs with HHT is their cortical location in all cases. It is reported that 77 (27.2%) of 283 sporadic AVMs were located in noncortical areas, including basal ganglia, thalamus, etc. (25). Most previous reports have not clearly defined the topography of AVMs in cases of HHT, although two reports support our observation that these AVMs are found on the brain surface (5, 26).
Micro AVMs are reported in 7% of patients with sporadic AVMs (27). In patients with HHT, we identified micro AVMs in seven of our 14 patients and in 12 (42.9%) of our 28 AVMs. Previous smaller case series have also reported the high incidence of micro AVMs in cases of HHT (12, 26, 27). Yasargil (28) introduced the term micro AVM and Willinsky et al (29) also reported that such lesions frequently present with hemorrhage. In our HHT cases, however, the micro AVMs never bled at the time of presentation nor during the follow-up period in those patients for whom no treatment had been performed (seven of 12 patients). This raises the question of the need for treatment of these micro AVMs.
The role of MR imaging in the diagnosis of cerebral AVM with HHT disorder is not established. Fulbright et al (30) reported that MR images could underestimate the prevalence of cerebral vascular malformations in patients with HHT. Ideally, contrast-enhanced high-resolution MR imaging of the entire brain should be performed for screening of patients with suspected HHT disorder. For practical reasons, we continue to screen such patients by using routine MR imaging, with the understanding that not all micro AVMs will be detected. Angiography, and possibly superselective angiography, is required to diagnose all AVMs, including micro AVMs in patients with HHT. In addition to the issue that multiple lesions should suggest the HHT diagnosis, it is also important to consider other clinical manifestations in detail, such as mucocutaneous telangiectasia, frequent episodes of epistaxis, gastrointestinal bleeding, etc. Children, in particular, with HHT do not always have these symptoms, although adults with HHT frequently do have them (7, 8, 10). The first clinical presentation of HHT in a child may be a cerebral AVM. This may result in the failure to make the diagnosis of HHT in children. DNA mutation analysis can provide a definitive diagnosis, although this is available only in certain research laboratories. This analysis is currently being conducted on some of our patients suspected of having HHT disorder.
We have described the angiographic and clinical characteristics of AVMs associated with HHT. The three distinct types of angioarchitecture might result in the different clinical manifestations. Further follow-up is required to establish whether these AVMs require a different treatment strategy. Although angiographic and clinical characteristics were identified in our HHT patient population, because of an inherent referral bias, they may not be representative of the HHT population at large.
Conclusion
The angiographic characteristics of the AVMs associated with HHT revealed that multiple cortical micro AVMs or AVFs are highly suggestive of HHT disorder. AVFs and small AVMs were frequently symptomatic, whereas the micro AVMs were usually asymptomatic at presentation.
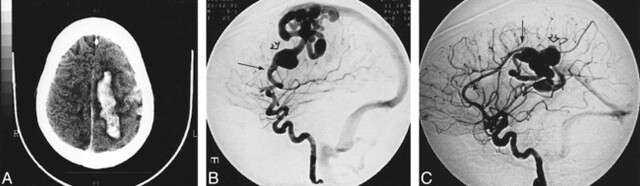
fig 2.
Images from the case of a 15-year-old woman who presented with right hemiplegia, a history of recurrent epistaxis, and a family history of HHT. She was also subsequently diagnosed with a pulmonary AVM.
A, Unenhanced CT scan shows an intracerebral hemorrhage and subarachnoid hemorrhage.
B, Lateral-view angiogram of the left internal carotid artery shows an AVF (arrow) involving the left frontal lobe cortex, arising from a middle cerebral artery branch, with cortical drainage and venous pouch (open arrow).
C, Lateral-view angiogram of the right internal carotid artery shows another AVF (arrow) involving the right parietal cortex supplied by the right pericallosal artery with deep drainage and venous pouch (open arrow).
Acknowledgments
We thank Drs. M.C. Wallace, M. Tymianski, and M. Schwartz for support of the University of Toronto Brain Vascular Malformation Study Group and Drs. J.T. Rutka, R.P. Humphreys, and D. Armstrong of the Hospital for Sick Children for allowing us access to their patients. We also thank Dr. R. Hyland of Wellesley/St. Michael Hospital for clinical support and Dr. R.I. White Jr. of Yale University for advice and encouragement regarding this manuscript. Finally, we acknowledge the editing efforts of Clayton Young from the University of Toronto.
Footnotes
Presented at the Scientific Meeting of HHT Foundation International, Inc., June 19−21, 1999, Denmark, and at the 11th Annual Meeting of the Neuroradiological Society, August 27−29, 1999, Montreal, Quebec, Canada.
Address reprint requests to Karel G. ter Brugge, MD, 399 Bathurst Street, Toronto, Ontario, Canada M5T 2S8.
References
- 1.Guttmacher AE, Marchuk DA, White RIJ. Hereditary hemorrhagic telangiectasia. N Engl J Med 1995;333:918-924 [DOI] [PubMed] [Google Scholar]
- 2.McAllister KA, Grogg KM, Johnson DW, et al. Endoglin, a TGF-B binding protein of endothelial cells, is the gene for hereditary hemorrhagic telangiectasia type I. Nat Genet 1994;8:345-351 [DOI] [PubMed] [Google Scholar]
- 3.Vincent P, Plauchu H, Hazan J, et al. A third locus for hereditary hemorrhagic telangiectasia maps to 12q. Hum Mol Genet 1995;4:945-949 [DOI] [PubMed] [Google Scholar]
- 4.Johnson DW, Berg JN, Gallione CJ, et al. A second locus for hereditary hemorrhagic telangiectasia mapped to chromosome 12. Genome Res 1995;5:21-28 [DOI] [PubMed] [Google Scholar]
- 5.Willinsky RA, Lasjaunias P, Ter Brugge K, Burrows P. Multiple cerebral arteriovenous malformations (AVMs): review of our experience from 203 patients with cerebral vascular lesions. Neuroradiology 1990;32:207-210 [DOI] [PubMed] [Google Scholar]
- 6.Ference BA, Shannon TM, White RIJ, Zawin M. Burdge CM. Life-threatening pulmonary hemorrhage with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia. Chest 1994;106:1387-1390 [DOI] [PubMed] [Google Scholar]
- 7.Wirth JA, Pollak JS, White RIJ. Pulmonary arteriovenous malformations. Curr Pulm Crit Care Med 1996;17:272-274 [Google Scholar]
- 8.Plauchu H, de Chadarévian JP, Bideau A, Robert JM. Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet 1989;32:291-297 [DOI] [PubMed] [Google Scholar]
- 9.Yasargil MG. Pathological consideration. In: Yasargil MG, ed. Microneurosurgery. vol 3A. Stuttgart: Thieme 1987 63-64
- 10.Jessurun GAJ, Kamphuis DJ, van der Zande FHR, Nossent JC. Cerebral arteriovenous malformations in the Netherlands Antilles: high prevalence of hereditary hemorrhagic telangiectasia-related single and multiple cerebral arteriovenous malformations. Clin Neurol Neurosurg 1993;95:193-198 [DOI] [PubMed] [Google Scholar]
- 11.Aesch B, Lioret E, de Toffol B, Jan M. Multiple cerebral angiomas and Rendu-Osler-Weber disease: case report. Neurosurgery 1991;29:599-602 [DOI] [PubMed] [Google Scholar]
- 12.Putman CM, Chaloupka JC, Fulbright RK, Awad IA, White RI Jr, Fayed PB. Exceptional multiplicity of cerebral arteriovenous malformations associated with hereditary hemorrhagic telangiectasia (Osler-Rendu-Weber syndrome). AJNR Am J Neuroradiol 1996;17:1733-1742 [PMC free article] [PubMed] [Google Scholar]
- 13.Coubes P, Humbertclaude V, Rodesch G, Lasjaunias P, Echenne B, Frerebeau P. Total endovascular occlusion of a giant direct arteriovenous fistula in the posterior fossa in a case of Rendu-Osler-Weber disease. Child Nerv Syst 1996;12:785-788 [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Monaco R, Taylor W, Rodesch G, et al. Pial arteriovenous fistula in children as presenting manifestation of Rendu-Osler-Weber disease. Neuroradiology 1995;37:60-64 [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi K, Kowada M, Tomura N, Kohkura H. Hereditary hemorrhagic telangiectasia associated with cerebral arteriovenous fistula and multiple cerebral arteriovenous malformations: case report. No Shinkei Geka 1994;22:85-91 [PubMed] [Google Scholar]
- 16.Kodoya C, Momota Y, Ikegami Y, Urasaki E, Wada S, Yokota A. Central nervous system arteriovenous malformations with hereditary hemorrhagic telangiectasia: report of a family with three cases. Surg Neurol 1994;42:234-239 [DOI] [PubMed] [Google Scholar]
- 17.John PR. Early childhood presentation of neurovascular disease in hereditary hemorrhagic telangiectasia. Pediatr Radiol 1992;22:140-141 [DOI] [PubMed] [Google Scholar]
- 18.Nakao S, Sukumitsu T, Yamamoto T, Sakamoto H. Cerebral and pulmonary arteriovenous fistula with possible hereditary hemorrhagic telangiectasia: case report. No Shinkei Geka 1986;14:901-906 [PubMed] [Google Scholar]
- 19.Eto RT, Harley JD, Chikos PM, Graebner RW. Subarachnoid hemorrhage in hereditary hemorrhagic telangiectasia: report of two cases. Neuroradiology 1974;8:127-130 [Google Scholar]
- 20.Waller JD, Greenberg JH, Lewis CW. Hereditary hemorrhagic telangiectasia with cerebrovascular malformations. Arch Dermatol 1976;112:49-52 [PubMed] [Google Scholar]
- 21.Adams HP Jr, Subbiah B, Bosch EP. Neurologic aspects of hereditary hemorrhagic telangiectasia: report of two cases. Arch Neurol 1977;34:101-104 [DOI] [PubMed] [Google Scholar]
- 22.Reddy K, West M, McClarty B. Multiple intracerebral arteriovenous malformations: a case report and literature review. Surg Neurol 1987;27:495-499 [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi T, Fujita S, Yamada H, Nishida Y, Izawa I. Multiple cerebral and pulmonary arteriovenous malformations associated with brain and subcutaneous abscesses: a possible variant of hereditary hemorrhagic telangiectasia, case report. Neurol Med Chir (Toyko) 1990;30:272-276 [DOI] [PubMed] [Google Scholar]
- 24.Yasargil MG. Microneurosurgery. vol 3A. Stuttgart: Thieme 1987 165-181
- 25.Viñuela F. Functional evaluation and embolization of intracranial arteriovenous malformation. In: Viñuela F, Halbach VV, Dion JE, eds. Interventional Neuroradiology: Endovascular Therapy of the Central Nervous System. New York: Raven Press 1992 77-86
- 26.Kikuchi K, Kowada M, Sasajima H. Vascular malformation of the brain in hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease). Surg Neurol 1994;41:374-380 [DOI] [PubMed] [Google Scholar]
- 27.Berenstein A, Lasjaunias P. Classification of CVMs. In: Surgical Neuroangiography. vol 4. Berlin: Springer 1992 25-80
- 28.Yasargil MG. Microneurosurgery.. vol 3B. Stuttgart: Thieme 1987 16-18
- 29.Willinsky R, Lasjaunias P, Comoy J, Pruvost P. Cerebral micro arteriovenous malformations (mAVM): review of 13 cases. Acta Neurochir (Wien) 1988;91:29-36 [DOI] [PubMed] [Google Scholar]
- 30.Fulbright RK, Chaloupka JC, Putman CM, et al. MR of hereditary hemorrhagic telangiectasia: prevalence and spectrum of cerebrovascular malformations. AJNR Am J Neuroradiol 1998;19:477-484 [PMC free article] [PubMed] [Google Scholar]