Abstract
BACKGROUND AND PURPOSE: Duplex sonography is an effective tool for evaluating internal carotid artery (ICA) stenosis, and power Doppler imaging has improved its value in this regard. Our goal was to elucidate which parameters, such as linear stenosis, area stenosis, and peak systolic velocity (PSV), are the most reliable predictors of ICA stenosis greater than 70% using the method proposed by the North American Symptomatic Carotid Endarterectomy Trial (NASCET).
METHODS: Duplex sonography with power Doppler imaging and cerebral angiography were performed prospectively in 75 patients (135 vessels). The grade of stenosis on angiograms was calculated by the NASCET method, and linear stenosis, area stenosis, and PSV were measured in the most stenotic part of the ICA.
RESULTS: Angiography revealed 20 ICA vessels with stenosis greater than 70%. The correlation between angiographic stenosis and linear stenosis, area stenosis, and PSV was .82, .78, and .84, respectively. A sensitivity-specificity curve analysis determined optimal threshold values of linear stenosis, area stenosis, and PSV as predictors of ICA stenosis greater than 70% as 74.7%, 83.3%, and 200 cm/s, respectively. Calculations of positive and negative predictive values, and accuracy using the optimal threshold values were 90.5%, 99.1%, and 97.8% for linear stenosis; 76.0%, 99.1%, and 94.8% for area stenosis; and 100%, 100%, and 100% for PSV.
CONCLUSION: All parameters corresponded relatively well with angiographic stenosis. In particular, PSV greater than 200 cm/s was the most reliable predictor of ICA stenosis greater than 70%. We believe that the combination of parameters plays a crucial role in the accurate assessment of ICA stenosis.
In 1991, the North American Symptomatic Carotid Endarterectomy Trial (NASCET) proved conclusively the effectiveness of carotid endarterectomy in patients with symptomatic internal carotid artery (ICA) stenosis greater than 70% (1). Although conventional cerebral angiography was used to assess carotid stenosis in the study, this technique carries a 0.5% to 5% risk of permanent neurologic deficits (2, 3). Therefore, duplex carotid sonography may be effective for evaluating the presence and severity of carotid stenosis to minimize these risks.
Peak systolic velocity (PSV) as measured by duplex sonography is a widely accepted and reliable diagnostic method for assessing ICA stenosis (4–10). Recently, color Doppler flow imaging has shown not only high accuracy but also excellent reproducibility in the assessment of carotid stenosis. Moreover, power Doppler imaging, the most recent sonographic innovation, generates intravascular color signals based on the reflected echo amplitude produced essentially from the amount of red blood cells within the sample volume. Thus, the color display on power Doppler images is independent of the angle of insonation and, more important, from the velocity and direction of moving blood cells, increased sensitivity displays continuity of the stenotic lumen. Therefore, power Doppler imaging more accurately depicts ICA stenosis by providing better visualization of the stenotic vascular lumen than does color Doppler flow imaging (11–14).
Discrepancies between the effectiveness of duplex sonography and angiography have been documented previously (9, 13, 15). To evaluate the degree of ICA stenosis by using duplex sonography with power Doppler imaging, such parameters as linear stenosis, area stenosis, and PSV are frequently used. The aim of this study was to elucidate which parameters are the most reliable for predicting ICA stenosis greater than 70% by the NASCET method (16).
Methods
Between May 1, 1997, and October 30, 1998, 75 patients (66 men and nine women; mean age, 63 years; range, 34–78 years) with cerebrovascular disease underwent sonographic examination with power Doppler imaging within 1 week before or after cerebral angiography. One ICA vessel could not be evaluated by cerebral angiography owing to technical difficulty, and duplex sonography could not be performed in five vessels because of inadequate insonation due to calcifications. Therefore, 144 vessels were examined in the present study.
Duplex sonography was performed by an experienced clinician using an ATL Ultramark 9 HDI (Advanced Technology Laboratories, Bothell, WA) unit with a linear-array pulsed wave transducer operating at 5.0 to 10.0 MHz. The pulse repetition frequency was primarily 5000 Hz, and the low-pass filter was 50 Hz. Imaging was performed while the subjects were lying in a supine position with the head turned away from the side being scanned and the neck extended. The ICA origin was examined in longitudinal and transverse planes using anterior, lateral, and posterior approaches by both B-mode imaging and power Doppler imaging. Using power Doppler imaging, we measured the degree of diameter stenosis in longitudinal views (linear stenosis) and the percentage of area reduction (area stenosis). Linear stenosis (%) was calculated as [1 − (r/n)] × 100, where r is the diameter of the residual lumen and n is the diameter of the normal vessel, using the longitudinal plane on power Doppler imaging. Area stenosis (%) was measured as [1 − (Ar/An)] × 100, where Ar represents the area of the residual lumen and An represents the area of the normal vessel.
To measure blood flow velocity on longitudinal scans, a5- to 7-mm sample was set in the ICA and displayed as linearly as possible. Special care was taken to maintain the incident angle between the ICA and the beam at 30° to 60°. PSV was measured bilaterally in the ICAs. When power Doppler images showed stenotic lesions in the ICA, the sample volume was moved slowly, proximally to distally in the ICA stenosis to obtain the highest flow velocity. These velocities were corrected using the incidence angle.
Selective cerebral angiography was performed by using the intraarterial digital subtraction technique via the femoral artery route with selective catheterization of the extracranial arteries. Biplanar images were obtained for each ICA. The neurologist who evaluated the angiograms was unaware of the results of power Doppler imaging. Linear-based methods of NASCET (15) were used to measure reduction of the ICA diameter. Using the NASCET method, we calculated correlations between the values of the sonographic parameters (area stenosis, linear stenosis, and PSV) and the degree of stenosis. In an analysis of PSV, we made a logarithmic transformation of PSV.
A sensitivity-specificity curve analysis was applied to obtain cutoff values for linear stenosis, area stenosis, and PSV for predicting ICA stenosis greater than 70%. In addition, we calculated the positive and negative predictive values and accuracy using the cutoff value of these parameters. Statistical significance was determined at the P < .05 level.
Results
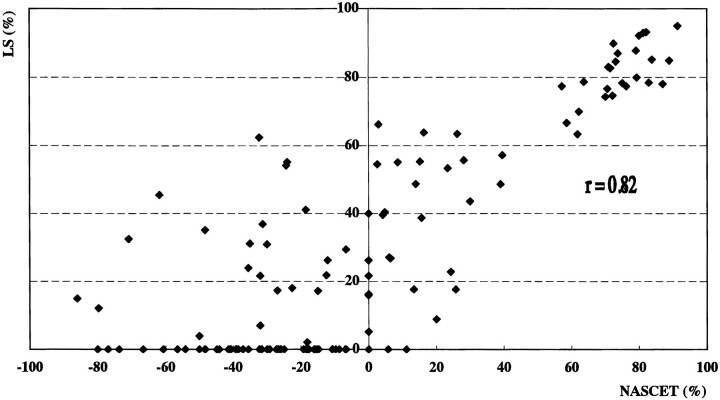
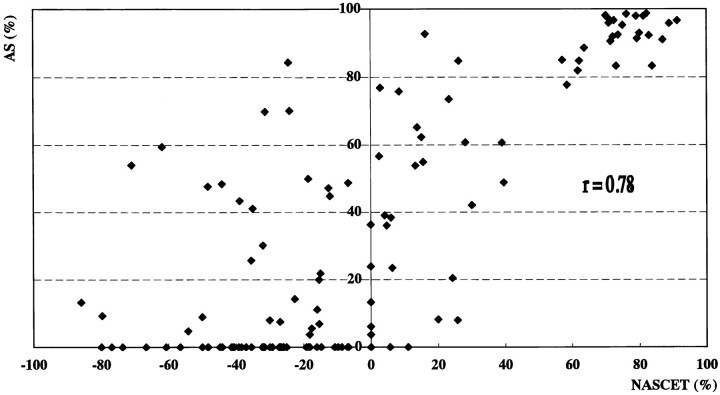
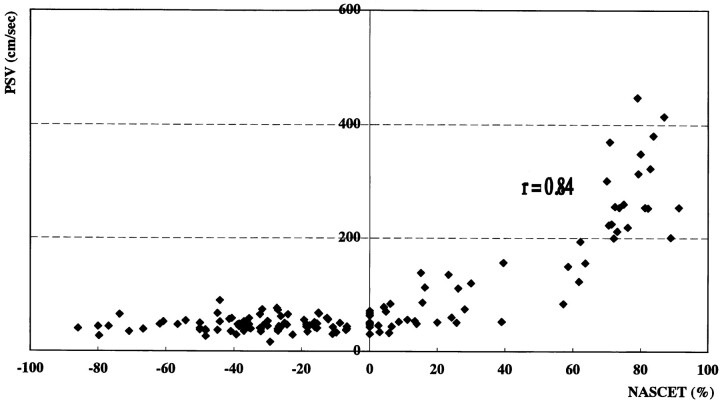
Angiography revealed 20 ICA vessels with stenosis greater than 70%, eight with stenosis greater than 30% but less than 70%, 107 that were normal or that had less than 30% stenosis, and nine that were occluded. Sonography with power Doppler imaging also showed these nine ICA occlusions. Nine vessels with ICA occlusion were excluded from the analysis. Figure 1 shows an angiogram, color and power Doppler images, and PSV in a patient with 70% ICA stenosis confirmed by angiography. The correlations between sonographic parameters and degree of stenosis on angiography were evaluated in the remaining 135 vessels (Figs 2–4). Correlations between stenosis revealed by the NASCET procedure and linear stenosis, area stenosis, and PSV were .82, .78, and .84, respectively (P < .0001).
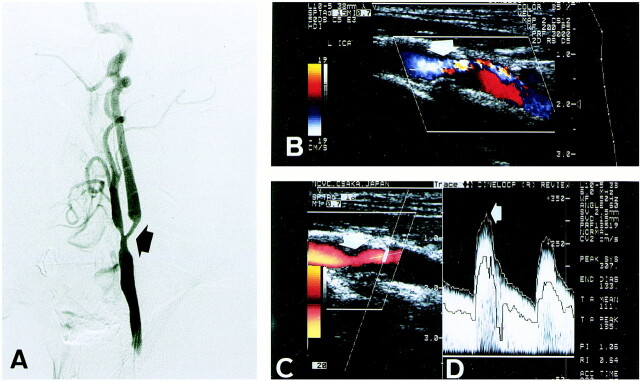
fig 1.
A, Lateral view of left common carotid angiogram shows 70% ICA stenosis by the NASCET method (arrow).
B, Color Doppler image shows severe ICA stenosis (arrow).
C, Power Doppler image shows 82% ICA stenosis by the linear stenosis parameter (arrow).
D, Flow velocity of poststenotic lesion indicates that PSV is 307 cm/s (arrow).
fig 2.
Scatterplot shows correlation between degree of stenosis by the NASCET method and linear stenosis (r = .82, P < .0001)
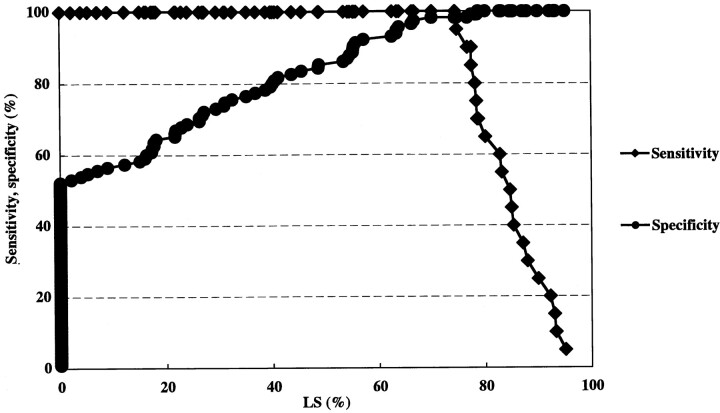
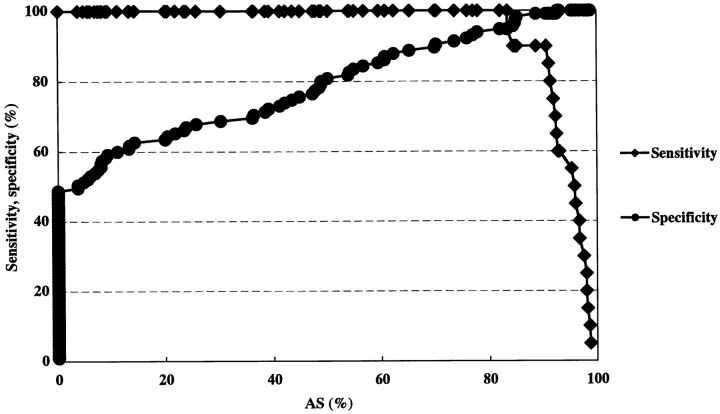
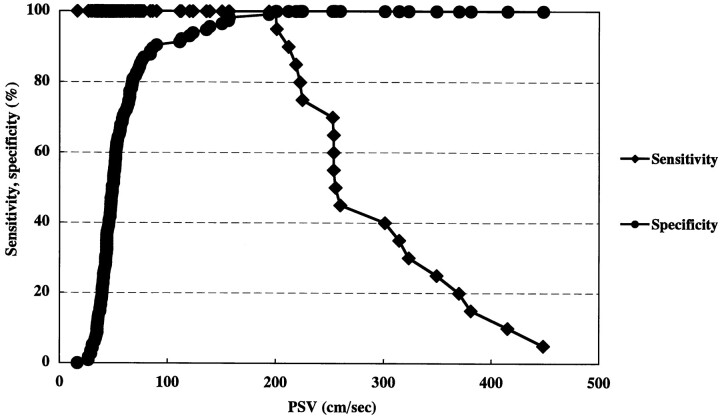
In the sensitivity-specificity curve analysis, optimal threshold values of linear stenosis, area stenosis, and PSV for predicting ICA stenosis greater than 70% were 74.7%, 83.3%, and 200 cm/s, respectively (Figs 5–7). Calculations of positive and negative predictive values and accuracy using the optimal threshold values were 90.5%, 99.1%, and 97.8% for linear stenosis; 76.0%, 99.1%, and 94.8% for area stenosis; and 100%, 100%, and 100% for PSV.
fig 5.
A sensitivity-specificity curve by linear stenosis. An optimal threshold value of linear stenosis for predicting ICA stenosis greater than 70% is 74.7%
Discussion
All parameters used in the present study corresponded relatively well to the degree of ICA stenosis as determined by the NASCET method. In particular, PSV greater than 200 cm/s was the most reliable predictor of ICA stenosis greater than 70%. The regression coefficients ranging from .78 to .84 in the present study may be higher than those reported previously, because a single, experienced clinician examined all patients and used the same equipment.
Steinke et al (13) suggested that power Doppler imaging has several advantages over color Doppler flow imaging in the diagnosis of carotid artery stenosis, and found only a moderate correlation between the NASCET angiographic method and the power Doppler imaging method. In the present study, PSV was also a more reliable predictive parameter than linear stenosis or area stenosis on power Doppler images. We believe that this finding is due to differences in the calculation of the degree of ICA stenosis between the NASCET angiographic method and the power Doppler imaging method. The degree of ICA stenosis calculated by the NASCET method is derived by dividing the diameter of the residual lumen by the diameter of the first normal distal segment of the distal ICA. Power Doppler imaging, on the other hand, directly measures the bulb of the ICA. The carotid bulb is always larger than that of the more distal ICA. Thus, the NASCET method consistently underestimates the degree of stenosis. In the present study, therefore, the degree of ICA stenosis as determined by the linear stenosis parameter using power Doppler imaging was often higher than that determined by the NASCET method.
The optimal threshold value of PSV for severe ICA stenosis ranged from 125 to 325 cm/s (4–10). In a report by the NASCET group, a PSV of 250 cm/s is quoted as a criterion for ICA stenosis greater than 70% (17). The PSV criterion for ICA stenosis greater than 70% reported herein is similar to that in previous reports (4–10, 17).
We recognize certain limitations in the present study. First, van Everdingen et al (15) reported that the accuracy in predicting the degree of ICA stenosis with PSV decreased when a contralateral high-grade stenosis or occlusion was present. In those cases, an increase in blood flow through the ICA to the contralateral middle cerebral artery via the anterior communicating artery caused an increased flow velocity in the ICA. Second, PSV peaks at about 90% ICA stenosis and then gradually decreases (18). Unfortunately, no such cases were observed in the present study. Our patients with unilateral ICA occlusion had no contralateral ICA stenosis greater than 70% nor a PSV less than 200 cm/s.
We used power Doppler images to select the sampling site of flow velocity. We might have selected a less than optimal sampling position within the vessel, where velocity of flow is not maximal, because flow signal is fairly uniform across the vessel lumen on power Doppler images. The optimal sampling location may be better pinpointed with color duplex flow imaging, which shows a variation of velocity by color change across the vessel lumen; however, this would have resulted in less optimal velocity measurements in those cases in which the maximal poststenotic velocity is not in the center of the lumen.
The American Heart Association guidelines on carotid endarterectomy published in 1995 stated that duplex sonography or duplex sonography combined with MR angiography may suffice in determining the severity of the extracranial portion of the ICA, although angiography remains the most reliable assessment of the precise degree of carotid artery stenosis (19). Patel et al (20) reported that combined use of three-dimensional time-of-flight MR angiography and duplex sonography resulted in a marked increase in accuracy to a level that obviates conventional angiography in the majority of patients. We believe that the use of sonography will reduce the need for conventional angiography in the future. Consequently, clinicians require accurate sonographic criteria to predict ICA stenosis greater than 70%.
On the basis of the findings in this study, we think that an evaluation of ICA stenosis by duplex sonography should initially involve the use of power Doppler imaging to detect the presence of stenotic lesions. Furthermore, clinicians should measure PSV at such stenotic lesions, and those with a PSV value greater than 200 cm/s should be considered to have a stenosis greater than 70%.
Conclusion
All parameters in the present study corresponded relatively well to ICA stenosis as detected by the NASCET method. In particular, PSV greater than 200 cm/s was the most reliable predictive parameter of ICA stenosis greater than 70%. We believe that the use of a combination of parameters—including linear stenosis and area stenosis on power Doppler images and PSV—plays a crucial role in the accurate assessment of the degree of ICA stenosis.
fig 3.
Scatterplot shows correlation between degree of stenosis by the NASCET method and area stenosis (r = .78, P < .0001)
fig 4.
Scatterplot shows correlation between degree of stenosis by the NASCET method and PSV (r = .84, P < .0001)
fig 6.
A sensitivity-specificity curve by area stenosis. An optimal threshold value of area stenosis for predicting ICA stenosis greater than 70% is 83.3%
fig 7.
A sensitivity-specificity curve by PSV. An optimal threshold value of PSV for predicting ICA stenosis greater than 70% is 200 cm/s
Footnotes
Supported in part by the Research Grant for Cardiovascular Disease (12A-2) from the Ministry of Health and Welfare of Japan, and by the Special Coordination Funds for Promoting Science and Technology (Strategic Promotion System for Brain Science) from the Science and Technology Agency of Japan.
References
- 1. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445-453 [DOI] [PubMed] [Google Scholar]
- 2.Hankey GJ, Warlow CP, Sellar RJ. Cerebral angiographic risk in mild cerebrovascular disease. Stroke 1990;21:209-222 [DOI] [PubMed] [Google Scholar]
- 3.Heiserman JE, Dean BL, Hodak JA, et al. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol 1994;15:1401-1407 [PMC free article] [PubMed] [Google Scholar]
- 4.Derdeyn CP, Powers WJ, Moran CJ, Cross DT III, Allen BT. Role of Doppler US in screening for carotid atherosclerotic disease. Radiology 1995;197:635-643 [DOI] [PubMed] [Google Scholar]
- 5.Hunink MG, Polak JF, Barlan MM, O'Leary DH. Detection and quantification of carotid artery stenosis: efficacy of various Doppler velocity parameters. AJR Am J Roentgenol 1993;160:619-625 [DOI] [PubMed] [Google Scholar]
- 6.Moneta GL, Edwards JM, Chitwood RW, et al. Correlation of North American Symptomatic Carotid Endarterectomy Trial (NASCET) angiographic definition of 70% to 99% internal carotid artery stenosis with duplex scanning. J Vasc Surg 1993;17:152-159 [DOI] [PubMed] [Google Scholar]
- 7.Faught WE, Mattos MA, van Bemmelen PS, et al. Color-flow duplex scanning of carotid arteries: new velocity criteria based on receiver operator characteristic analysis for threshold stenoses used in the symptomatic and asymptomatic carotid trials. J Vasc Surg 1994;19:818-828 [DOI] [PubMed] [Google Scholar]
- 8.Neale ML, Chambers JL, Kelly AT, et al. Reappraisal of duplex criteria to assess significant carotid stenosis with special reference to reports from the North American Symptomatic Carotid Endarterectomy Trial and the European Carotid Surgery Trial. J Vasc Surg 1994;20:642-649 [DOI] [PubMed] [Google Scholar]
- 9.Ranke C, Creutzig A, Alexander K. Duplex scanning of the peripheral arteries: correlation of the peak velocity ratio with angiographic diameter reduction. Ultrasound Med Biol 1992;18:433-440 [DOI] [PubMed] [Google Scholar]
- 10.Robinson ML, Sacks D, Perlmutter GS, Marinelli DL. Diagnostic criteria for carotid duplex sonography. AJR Am J Roentgenol 1988;151:1045-1049 [DOI] [PubMed] [Google Scholar]
- 11.Steinke W, Meairs S, Ries S, Hennerici M. Sonographic assessment of carotid artery stenosis: comparison of power Doppler imaging and color Doppler flow imaging. Stroke 1996;27:91-94 [DOI] [PubMed] [Google Scholar]
- 12.Griewing B, Morgenstern C, Driesner F, Kallwellis G, Walker ML, Kessler C. Cerebrovascular disease assessed by color-flow and power Doppler ultrasonography: comparison with digital subtraction angiography in internal carotid artery stenosis. Stroke 1996;27:95-100 [DOI] [PubMed] [Google Scholar]
- 13.Steinke W, Ries S, Artemis N, Schwartz A, Hennerici M. Power Doppler imaging of carotid artery stenosis: comparison with color Doppler flow imaging and angiography. Stroke 1997;28:1981-1987 [DOI] [PubMed] [Google Scholar]
- 14.Schmidt P, Sliwka U, Simon SG, Noth J. High-grade stenosis of the internal carotid artery assessment by color and power Doppler imaging. J Clin Ultrasound 1998;26:85-89 [DOI] [PubMed] [Google Scholar]
- 15.van Everdingen KJ, van der Grond J, Kappelle LJ. Overestimation of a stenosis in the internal carotid artery by duplex sonography caused by an increase in volume flow. J Vasc Surg 1998;27:479-485 [DOI] [PubMed] [Google Scholar]
- 16. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Steering Committee. North American Symptomatic Carotid Endarterectomy Trial: methods, patient characteristics, and progress. Stroke 1991;22:711-720 [DOI] [PubMed] [Google Scholar]
- 17.Eliasziw M, Rankin RN, Fox AJ, Haynes RB, Barnett HJM, for the North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group Accuracy and prognostic consequences of ultrasonography in identifying severe carotid artery stenosis. Stroke 1995;26:1747-1752 [DOI] [PubMed] [Google Scholar]
- 18.Jacobs NM, Grant EG, Schellinger D, Byrd MC, Richardson JD, Cohan SL. Duplex carotid sonography: criteria for stenosis, accuracy, and pitfalls. Radiology 1985;154:385-391 [DOI] [PubMed] [Google Scholar]
- 19.Moore WS, Barnett HJM, Beebe HG, et al. Guidelines for carotid endarterectomy: a multidisciplinary consensus statement from the ad hoc committee, American Heart Association. Stroke 1995;26:188-201 [DOI] [PubMed] [Google Scholar]
- 20.Patel MR, Kuntz KM, Klufas RA, et al. Preoperative assessment of the carotid bifurcation: can magnetic resonance angiography and duplex ultrasonography replace contrast arteriography? Stroke 1995;26:1753-1758 [DOI] [PubMed] [Google Scholar]