Abstract
BACKGROUND AND PURPOSE: Our long-term goal is to improve intraaneurysmal fibrosis after aneurysm embolization, by implanting exogenous fibroblasts, using platinum coils. For the current project, we tested two hypotheses: 1) that exogenous, fluorescence-labeled rabbit fibroblast allografts remained viable and proliferated within rabbit carotid arteries, and 2) that these fibroblast allografts could be reliably implanted into experimental aneurysms by use of platinum coils.
METHODS: Part 1. New Zealand White rabbit synovial fibroblasts obtained from a commercial vender were labeled with a fluorescent membrane marker. The common carotid arteries of New Zealand White rabbits were surgically exposed, ligated proximally and distally, and entered with 22-g angiocatheters. Through the angiocatheter we injected either phosphate-buffered saline-containing fluorescence-labeled fibroblasts (treatment vessels) or saline only (control vessels). The wounds were closed, and the subjects were kept alive for various time points up to 2 weeks. After sacrifice, the carotid artery segments were resected, processed for frozen-section histologic examination, and evaluated using epifluorescent microscopy and hematoxylin and eosin staining. Cell viability and proliferation were determined by comparing the treatment versus control vessels.
Part 2. A) Fluorescence-labeled cells were grown in culture on platinum coils, which were then exposed to systemic arterial flow in the rabbit thoracic aorta for various lengths of time up to 40 minutes. The coil segments were then examined using fluorescent microscopy and the presence and relative amount of cells remaining on the coil were documented.
B) Experimental aneurysms in rabbits were embolized with control platinum coils (n = 9) and platinum coils bearing rabbit synovial fibroblasts that were grown onto the coils in culture prior to implantation (n = 9). Subjects were sacrificed 3, 7, and 14 days after coil implantation. Histologic samples were studied to assess the presence or absence of nucleated cells within and around coil winds in order to determine whether fibroblasts had been successfully implanted into aneurysms. Data were evaluated using the chi-square test for statistical significance.
RESULTS: Part 1. Fluorescence-labeled cells were examined in the treatment carotid artery segments and results were recorded at all time intervals. The treatment vessel segments showed evidence of progressive cellular proliferation, leading to complete vessel fibrosis at 2 weeks. Conversely, control vessel segments were filled predominately with unorganized thrombus at each time interval.
Part 2. A) Numerous labeled fibroblasts remained adherent to the coil despite prolonged exposure to systemic arterial flow.
B) Fibroblasts were seen adjacent to or within the central lumen of coils in eight (88%) of nine aneurysms treated with cell-bearing coils. Nucleated cells were not present in any of the nine control coil subjects. This represented a statistically significant difference (P < .001).
CONCLUSION: Fibroblast allografts remain viable and proliferate in the vascular space in rabbits. Furthermore, these same fibroblasts, after seeding onto platinum coils in culture, remain protected within the lumen of the coils and are retained within the coil lumen even after prolonged exposure to arterial blood flow. Coils can be used to deliver viable fibroblasts directly into experimental aneurysms successfully. These findings indicate that coil-mediated cell implantation is feasible and may be a potential method of increasing the biological activity of embolic coils.
Endovascular treatment of intracranial aneurysms by use of detachable coils is rapidly gaining acceptance as a safe alternative to standard surgical therapy (1–4). Despite the recent dramatic advances that have occurred in coil technology, several important shortcomings with endovascular therapy remain. Treatment of large (15–25 mm diameter) and giant (>25 mm) aneurysms has been problematic owing to low rates of initial complete aneurysm obliteration as well as high rates of late aneurysm recanalization (4, 5). Treatment outcomes for small and medium-sized aneurysms (<10 mm) have been more encouraging, but long-term studies suggest that a significant portion of treated aneurysms undergo partial regrowth secondary to coil compaction (6). The mechanisms responsible for aneurysm recanalization and coil compaction remain unknown, but limited human histologic data suggest that the thrombus induced by coil placement remains unorganized and that few fibroblasts are present within the thrombus (7).
Previous investigators have suggested that the inert nature of the platinum used to construct the Guglielmi detachable coil (GDC) may explain the lack of thrombus organization (8, 9). On the basis of this theory, many investigators have proposed modifications aimed at increasing the coil's “biological activity,” defined as improvement in thrombus organization, collagen formation, or endothelialization within and adjacent to the aneurysm cavity (9–15). Coils have been modified by additions of polymer coatings, basement membrane coatings, collagen filaments, and ion implantation. These modifications, however, do not address the lack of fibroblasts present within GDC-embolized aneurysms.
We hypothesize that endovascular delivery of fibroblasts directly to aneurysms by use of platinum coils as delivery vehicles will improve intraaneurysmal fibrosis and thus diminish aneurysm recanalization. The purpose of the current work is to establish 1) that exogenous, fluorescence-labeled rabbit fibroblast allografts remain viable and proliferate within rabbit carotid arteries, and 2) that these fibroblast allografts can be reliably implanted into experimental aneurysms by use of platinum coils as delivery vehicles.
Methods
Part 1. Cell Implant Viability and Proliferative Capacity
Cell Culture
HIG-82 rabbit synovial fibroblasts (American Type Culture Collection, Rockville, MD) were grown in Dulbecco's Modified Eagle Medium (D-MEM; GIBCO Life Technologies, Gaithersburg, MD) with 10% fetal bovine serum (HyClone Laboratories, Logan UT), penicillin (100 units/ml) (Sigma Chemical Company, St. Louis, MO), streptomycin (0.1 mg/ml) (Sigma), and amphotericin B (25 micrograms/ml) (Sigma). After a confluent cell layer was obtained, the monolayer was dissociated using trypsin, and cells were diluted to the seeding concentration by centrifugation and resuspension.
Fluorescent Labeling
In this experiment, we intended to follow proliferation in vivo of implanted cells. As such, it was necessary to label the implanted cells specifically in order to distinguish these cells and their progeny from reactive (host) cells. Cells were labeled with a commercially available fluorescence dye, PKH26, (Sigma) per the manufacturer's instructions. PHK26 is a lipophilic fluorescent dye that binds irreversibly to the cell membrane. It does not leak into the surrounding tissue and is not transferable to other cells (16–18). Briefly, cells were put in suspension in a concentration of 1 × 106 cells/ml. One ml of Diluent C (Sigma) was added, followed by 4 × 106 molar PKH26 dye. Three minutes after labeling, 2 ml of bovine serum was added and the mixture was incubated for 60 seconds. The cells were centrifuged and the supernatant aspirated to remove unattached label. The cells were washed three times with media, centrifuged, then resuspended in phosphate buffered solution (PBS) in preparation for implantation
In Vivo Experiments
The right carotid arteries of eight New Zealand white rabbits (treatment vessels [n = 4], control vessels [n = 4]) were surgically exposed, and isolated segments were formed by suture occlusion of 2-cm lengths of vessel. After surgical exposure of the carotid artery, the lumen was entered with a 24-g angiocatheter. Either labeled fibroblasts (approximately 1 × 105 cells suspended in 0.1 ml of PBS) or 0.1 ml of PBS were injected into the isolated vessel segments. Treatment (labeled, fibroblast-injected) and control (PBS-injected) vessel segments were harvested at 1 hour (treatment [n = 1], control [n = 1]), 3 days (treatment [n = 1], control [n = 1]), 7 days (treatment [n = 1], control [n = 1]), and 14 days (treatment [n = 1], control [n = 1]). The segments were rapidly frozen and cut into 20-μ sections. Every third section was stained with hematoxylin and eosin (H&E). Adjacent sections were evaluated for the presence of labeled cells by use of epifluorescent microscopy. A reader experienced in the interpretation of vascular histology (J.G.S.), blinded to the vessel type, examined H&E-stained sections from the middle portion of each sample. This reader assigned an estimated percent of lumen occlusion on the basis of degree of cellular infiltration and fibrosis. The same reader examined each section under epifluorescent microscopy for the presence of fluorescence-labeled cells.
Part 2: A. Adherence of Cells to Coils under Arterial Flow Conditions
Background
The goal of this portion of the study was to establish that cells cultured ex vivo onto GDC coils remain adherent during prolonged exposure to systemic arterial blood flow and, thus, can be successfully delivered to potential treatment sites. This hypothesis was tested by exposing GDC coils containing fluorescence-labeled cells to arterial flow in the rabbit thoracic aorta for successive time intervals and documenting the continued presence of labeled cells.
Detailed Methods
Unmodified GDC devices were sterilized and placed in a standard culture dish apparatus containing the previously described culture media. Fluorescence-labeled cells were deposited directly on each GDC coil. The GDC coil/cell suspension was placed in an incubator and viewed daily by using the inverted microscope until confluent cells were evident along the surface of the coil (Fig 1). At this time, the GDCs were retracted into the plastic coil housing in preparation for implantation into rabbits.
fig 1.
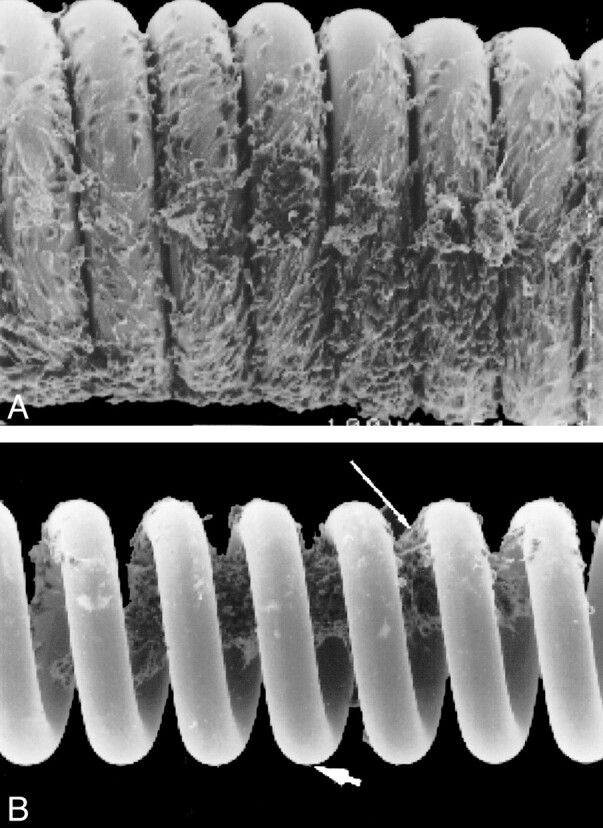
Electron microscopy image of GDC coils co-cultured with fibroblasts. Note fibroblasts growing on surface (A) and within the central lumen (B) of the coils
A single rabbit was anesthetized with an intramuscular injection of ketamine/xylazine (60/6 mg/kg, respectively), and the right femoral artery was surgically exposed. The artery was ligated distally using a 4–0 silk suture, and a 22-g angiocatheter was advanced in a retrograde direction into the artery. A 0.018-in guidewire was passed through the angiocatheter, and serial dilations of the artery were performed prior to placement of a 4F vascular sheath. Heparin (100 U/kg) was administered intravenously. The cell-bearing GDC coil was passed through a Tracker-10 microcatheter, and a 5-mm segment of the tip of the coil was removed so that it could be used as a preexposure control. Subsequently, the cell-bearing GDC was placed in the thoracic aorta and the distal 5-mm coil segment was pushed out of the catheter tip and exposed to arterial flow for 1-minute, 5-minute, 10-minute, 20-minute, and 40-minute intervals. After each time interval, the entire coil was retracted, and the distal 5-mm segment, which had been exposed to arterial blood flow, was severed and placed in 2% paraformaldehyde. The harvested coil segments were then examined using fluorescent microscopy for the presence and relative density of labeled cells.
B. Feasibility of Coil-mediated Implantation of Fibroblasts into Experimental Aneurysms
Aneurysm Creation
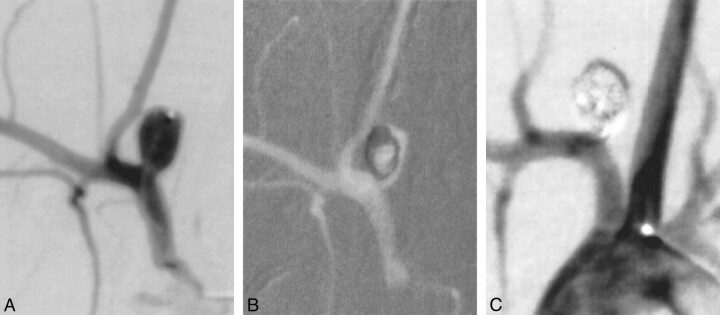
Saccular aneurysms were created in 18 New Zealand White rabbits (control [n=9], test subjects [n=9]) by using the technique of vessel ligation combined with intraluminal elastase incubation, as described in detail in previously published works (19). The arterial bifurcation of the right subclavian artery and right common carotid artery (CCA) was used to create a right CCA bifurcation aneurysm.
Anesthesia was induced with an intramuscular injection of ketamine/xylazine (60/6 mg/kg, respectively), followed by maintenance anesthesia with intravenous pentobarbital. Using sterile technique, surgical exposure of the right CCA was performed. Proximal and distal control of the vessel was obtained using a 4-0 silk suture. A 1- to 2-mm, beveled arteriotomy was made, and a 4F vascular sheath (AVANTI; Cordis Endovascular Systems, Miami Lakes, FL) was passed in a retrograde direction to the middle portion of the CCA. A 3F Fogarty balloon (Baxter Healthcare Corp., Irvine, CA) was advanced through the sheath to the level of the CCA origin and inflated with iodinated contrast material. Porcine elastase (Worthington Biochemical Corp., Lakewood, NJ) was incubated within the lumen of the right CCA above the Fogarty balloon for 20 minutes, after which the catheter system was removed and the vessel ligated in its middle portion. The skin was closed with a running suture. Animals were allowed to recover. Aneurysms were allowed to mature for at least 14 days prior to embolization. The aneurysm cavities measured approximately 3 to 5 mm in diameter and 4 to 6 mm in height.
Coil Embolization Procedure
Right common femoral artery access in the rabbits was achieved as described previously herein. A 4F catheter was advanced into the ascending aorta. Using coaxial technique, with a continuous heparinized saline flush, a Tracker-10 2-marker microcatheter (Target Therapeutics, Freemont, CA) was advanced into the aneurysm (Fig 2). The size of the aneurysm was assessed using direct comparison to radiopaque sizing devices. The aneurysms were embolized with either a control (unmodified) or treatment (cell-bearing) coil. Previous investigations in our laboratory have determined that electrolysis has a deleterious effect on cell growth (unpublished data). Thus, the fibroblast-coated GDCs were deployed using a coil pusher device rather than the standard electrolytic detachment typically used for GDC coils. After coil embolization, the catheters were removed. The vascular sheath was removed, and the proximal aspect of the femoral artery was ligated with a 4–0 silk suture. The skin was closed with a running suture. Animals were allowed to recover and were sacrificed at 3 days (control [n = 3], treatment [n = 3]) 7 days (control [n = 3], treatment [n = 3]), and 2 weeks (control[n = 3], treatment [n = 3]) after embolization.
fig 2.
A, Experimental aneurysm accessed with microcatheter.
B, Coils deployed under roadmap fluoroscopy.
C, Control angiogram shows complete occlusion of the aneurysm.
Histologic Preparation
At the time of sacrifice, the subjects were deeply anesthetized and then euthanized using an intracardiac injection of pentobarbital. The mediastinum was dissected, and the aortic arch and proximal great vessels, including the coil-embolized segment of artery, were exposed and dissected free from surrounding tissues. The tissue was immediately placed in formalin. After fixation for at least 24 hours, samples were embedded in methylmethacrylate, sectioned with a tungsten carbide knife at 30-μm increments, and stained with H&E.
Histologic Evaluation
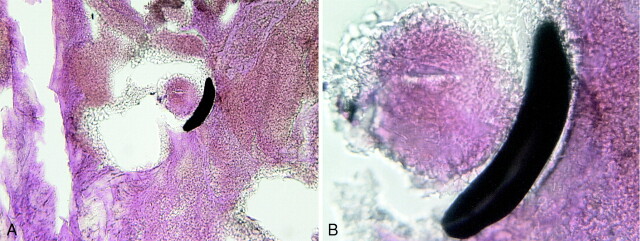
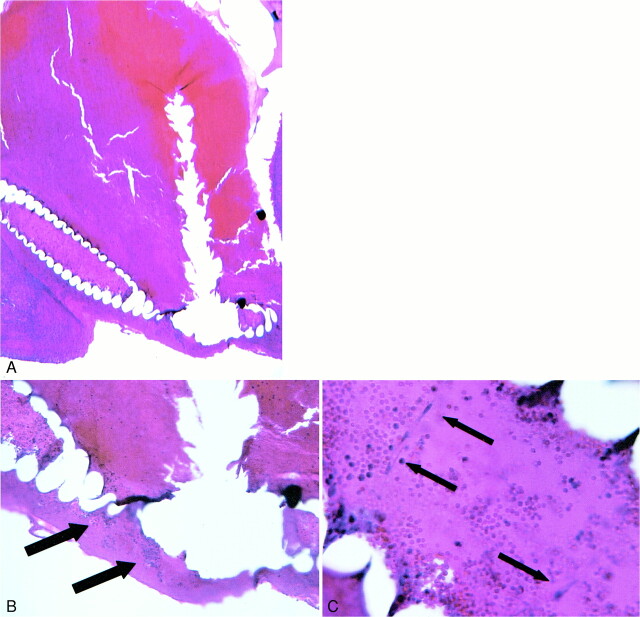
The purpose of this study was to establish the feasibility of implantation of fibroblast tissue allografts into aneurysms by use of platinum coils as delivery vehicles. The presence of implanted fibroblast tissue allografts was determined using histologic evaluation of the local cellular environment within and immediately adjacent to the coils (“pericoil histology”). Previous experiments have demonstrated that the histopathologic characteristics of coil-embolized aneurysms created using control coils are characterized by the presence of blood products, specifically nonnucleated red blood cells (RBCs), around and within the implanted coil loops (Fig 3) (20). In contrast, fibroblast tissue allografts demonstrate markedly different staining characteristics as compared with RBCs (Figs 4–6). Specifically, fibroblasts are nucleated and demonstrate basophilic cytoplasmic staining. Fibroblasts can be distinguished from other nucleated inflammatory cells such as lymphocytes or polymorphonuclear leukocytes by their elongated, spindled morphologic characteristics.
fig 3.
A, 14-day H&E-stained control aneurysm filled with unorganized thrombus.
B, 100× magnification shows coil wind surrounded only by RBCs.
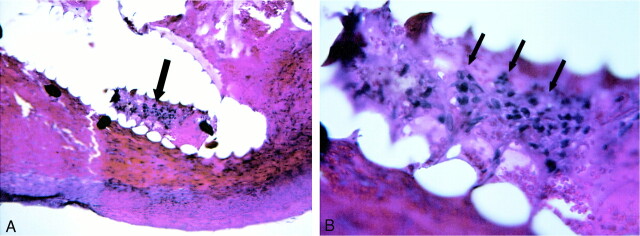
fig 4.
A, 40× H&E-stained 3-day sample showing coil bridging aneurysm neck.
B, 100× H&E-stained slide demonstrating nucleated cells within coil interstices (arrows) adjacent to endothelialized aneurysm neck.
C, 400× magnification shows fibroblasts (arrows) present within central lumen of coil.
Histologic findings were graded on a five-point scale on the basis of the cellular environment immediately adjacent to the implanted coils (Table). We focused on the histologic characteristics of the cells within the central lumen of the GDC coils and within the interstices of the primary coil winds (ie, along the outer surface of the coil). Whenever possible, portions of the sample containing intact coil fragments were examined. During histologic preparation, however, the coils were often dislodged from the specimens. In these cases, the previous location of the coils within the lumen was evident owing to the presence of a distinctively scalloped impression in the adjacent material (Figs 4–6).
Samples were evaluated in a blinded fashion by two experienced observers (D.F.K.,W.F.M.). Cases of disparate readings were viewed jointly, and a consensus reading obtained. Results were analyzed using the chi-square test for statistical significance.
Results
Part 1. Cell Implant Viability and Proliferative Capacity
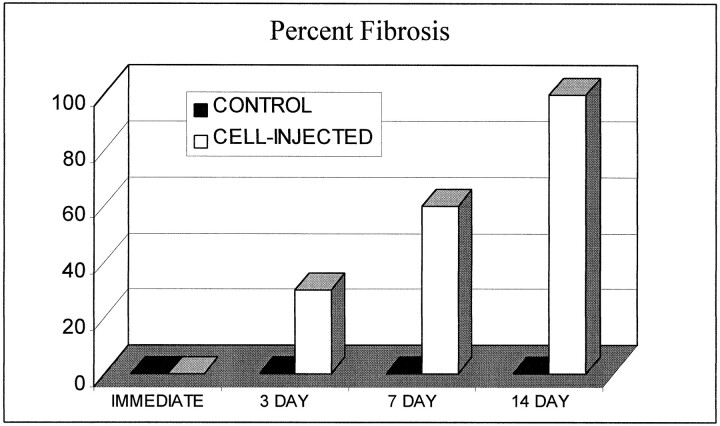
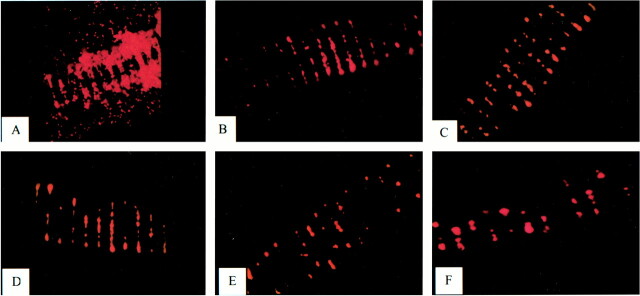
Fluorescent cells with the characteristic histologic appearance of fibroblasts were present in all of the treatment vessel segments and none of the control vessel segments (Figs 7 and 8). The estimated percent fibrosis of the treatment and control vessels at the various time points is demonstrated in Figure 9. The control group vessel segments were filled predominately with unorganized thrombus containing a scant cellular infiltrate. The treatment vessels had evidence of organizing fibroblast proliferation at 3 days. This progressed to complete obliteration of the vessel lumen, with organized cellular infiltrate at the 14-day data point.
fig 7.
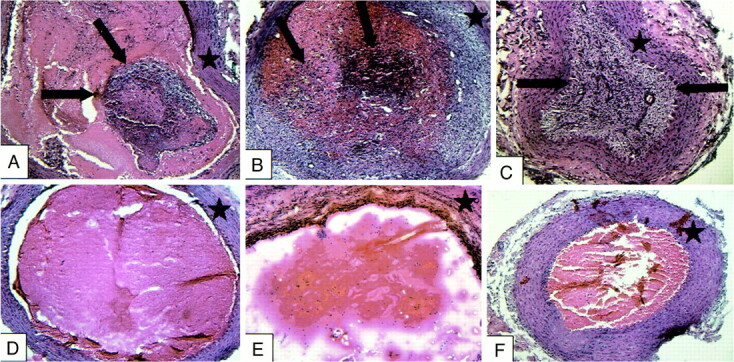
Treatment vessels containing implanted fibroblasts (A–C) and control vessels (D–F) at 3-day (A and D), 7-day (B and E), and 14-day (C and F) intervals. Treatment vessels show progressive intraluminal cellular proliferation and fibrosis (arrows). Control vessels are filled with unorganized thrombus with scant cellular infiltration. Star indicates vessel wall
fig 9.
Estimated percent fibrosis of the treatment and control vessels at each time interval. There was no organized fibrosis in the control vessels at any time point. The treatment vessels had fibroblast proliferation at 3 days, progressing to complete fibrosis of the vessel lumen at 14 days
Part 2
A. Feasibility of Coil-mediated Cell Delivery
Numerous fluorescence-labeled fibroblasts were seen within the interstices and within the central lumen of the GDC coils at all time intervals (Fig 10). Prior to withdrawing the coil into its plastic housing, extensive amounts of fibroblasts were seen on the surface and in the inner lumen of the GDC coils (Fig 10A). After the coil had been withdrawn into its plastic housing, the surface fibroblasts were removed, but cells present within the interstices and inner lumen of the coil remained (Fig 10B). Numerous cells remained on the coil at all time points (Fig 10B–F). The relative amount of cells present after 20 and 40 minutes in arterial flow is slightly diminished compared with the earlier time points.
fig 10.
Segments of GDC coils co-cultured with fluorescence-labeled fibroblasts.
A, Fluorescent cells in culture adherent to the surface of the coil and extending into interstices of the coil lumen.
B, Immediately after withdrawal of coil into housing, the cells on the surface are stripped. Cells remain within coil lumen. Coils exposed to systemic arterial flow for 5 minutes (C), 10 minutes (D), 20 minutes (E), and 40 minutes (F). Numerous cells remain at all time intervals. The density of cells is diminished at the 20- and 40-minute time points relative to earlier samples.
B. Coil-mediated Intraaneurysmal Fibroblast Implantation
Three-day Data
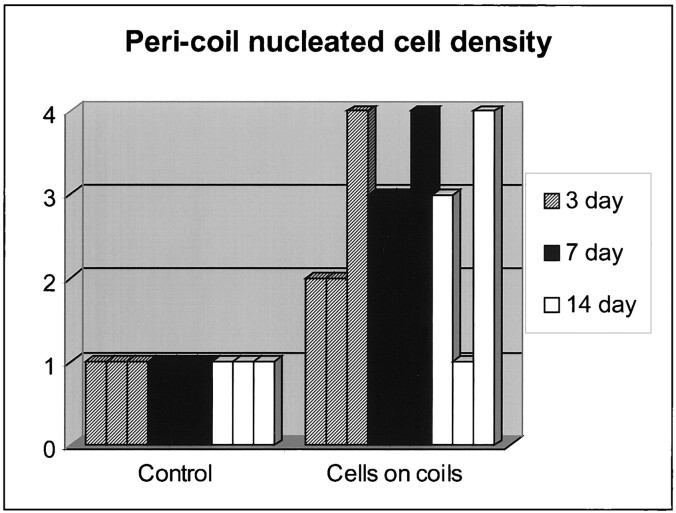
The pericoil histologic findings of the control coils at 3 days showed only RBCs immediately adjacent to the coil winds (Grade 1, n = 3) (Fig 3). All of the fibroblast-coated coils had nucleated cells within or immediately adjacent to the coil winds (Grade 2, n = 2; Grade 4, n = 1) (Figs 4 and 11).
Seven-day Data
All of the control samples were characterized by RBCs immediately adjacent to the coil winds (Grade 1, n = 3). All of the fibroblast-coated coils had nucleated cells within the interstices of the coil that extended into the tissue immediately surrounding the coil winds (Grade 3, n = 2; Grade 4, n = 1) (Fig 5).
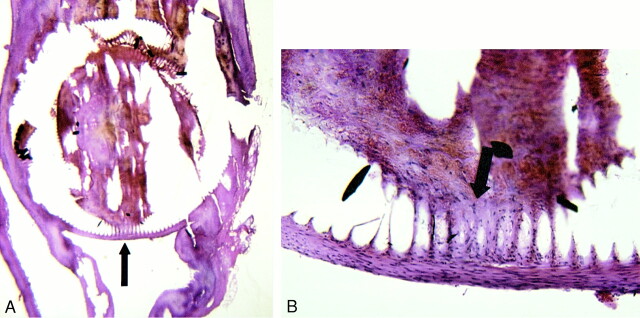
fig 5.
A, 100× H&E-stained 7-day sample. The arrow marks tissue that was present within the central lumen of the implanted coil. The basophilic, nucleated cells in the central region (arrow) are transplanted fibroblasts.
B, 400× magnification demonstrates fibroblasts (arrows) present within central lumen of coil.
Two-week Data
None of the control samples had evidence of nucleated cells within or adjacent to the coil winds (Grade 1, n = 3). Two of the fibroblast-coated coil samples had nucleated cells growing around the coil winds (Grade 3, n = 1; Grade 4, n = 1) (Fig 6). One of the experimental samples had no evidence of nucleated cells associated with the coil (Grade 1, n = 1). This sample was associated with technical difficulties, in which the coil winds herniated out of the neck of the aneurysm into the adjacent parent artery.
fig 6.
A, 40× H&E-stained view of 14-day speciment embolized with fibroblast-bearing GDC coil. The coil bridged the aneurysm neck, which developed neoendothelial lining.
B, 100× view of same specimen illustrates fibroblasts (arrow) present within the coil wind interstices and extending into the adjacent thrombus and aneurysm neck.
Summary of Results
Part 1: Fluorescence-labeled cells were observed in the treatment carotid artery segments at all time intervals, and findings were documented. The treatment vessel segments showed evidence of progressive cellular proliferation, leading to complete vessel fibrosis at 2 weeks.
Part 2: A) Numerous labeled fibroblasts remained adherent to the coil despite prolonged exposure to systemic arterial flow. B) Fibroblasts were seen adjacent to or within the central lumen of coils in eight (88%) of nine aneurysms treated with cell-bearing GDCs. Nucleated cells were present in none of the nine control coil subjects. This represented a statistically significant difference (P < .001).
Discussion
This study represents a significant advance in the preclinical evaluation of cell implantation technology for treatment of neurovascular disease. The data presented herein demonstrate that fibroblast allografts remain viable and proliferate in the vascular space in rabbits. Furthermore, these same fibroblasts, after seeding onto platinum coils in culture, remain protected within the lumen of the coils and are retained within the coil lumen even after prolonged exposure to arterial blood flow. Finally, these data indicate that cells can reliably be implanted into experimental aneurysms by use of platinum coils. All of these findings suggest that cell implantation techniques may one day enable increased rates of complete cure of cerebral aneurysms through the application of biologically modified coil technologies.
Our group has previously reported preliminary data regarding cell implantation (13). Our earlier work was limited to experimentation using nude rats. The choice of these immunoincompetent animals was made in order to avoid difficulties in rejection of the implanted cells by the recipients. Compared to our prior work, the current study more closely mimics the clinical scenario by the use of immunocompetent animals. Furthermore, the data presented herein greatly advance our understanding of the biology of the cell implants. Through the use of fluorescence-labeled cells, we were able to prove that the cells proliferating in the carotid arteries of the rabbits were indeed those cells we had implanted rather than cells reacting to the implants.
Another limitation of our prior work was that the rat data were limited to direct, surgical implantation of coil fragments. Full-length catheters, intact coils, and high-flow arterialized blood were not present in our previous experiments. The current work has demonstrated that commercially available catheter systems and modified, commercially available coils can be used to achieve reliable cell implantation, even in the face of arterial blood flow around the coils for prolonged periods. We also applied a realistic animal model in this study, wherein the morphologic characteristics of the aneurysm closely simulate those of human aneurysms (7, 20, 21). This reliable deposition of cells is enabled by the growth of cells into the central core of the platinum microcoils, wherein the cells are protected from being dislodged by blood flow.
Other authors have proposed cell implantation for improvement of aneurysm occlusion. Raymond et al have demonstrated, using a canine model of gelfoam implants, that ex vivo growth of activated smooth muscle cells onto embolic devices may improve aneurysm fibrosis (22). There are several important differences between our work and that of Raymond. First, the experiments reported by Raymond et al used surgical implantation of gelfoam sponges, whereas we performed endovascular cell delivery applying standard microcatheters and modified, commercially available platinum coils. Second, Raymond et al implanted smooth muscle cells into experimental aneurysms, whereas we chose to implant fibroblasts. There are several theoretical advantages of using fibroblasts rather than smooth muscle cells. Fibroblasts rapidly proliferate in culture, are extremely hardy, and thus are a very frequently used cell line in ex vivo gene therapy and cell transplantation work (23, 24). Smooth muscle cells, particularly human vascular smooth muscle cells, can be difficult to grow, which has limited their use in cell culture studies (25). Many investigators in the field of tissue engineering choose to use fibroblasts because of their limited ability to stimulate a significant immune response (24, 26). This is thought to be related to their non-expression of class II MHC antigens and their lack of as-yet-undefined co-stimulatory factors that allow a host to recognize an implanted cell as foreign (26). A tissue-engineered, Food and Drug Administration–approved artificial skin containing fibroblast allografts harvested from neonatal skin samples is currently available for treatment of nonhealing ulcers and other cutaneous defects (27–31). Little or no deleterious immune response to the implanted cells has been documented with this device (29). The relative immunogenicity of smooth muscle cell allografts is uncertain and, to our knowledge, no tissue-engineered product containing smooth muscle cells is currently available.
Numerous authorities have recognized the need for improved “biological activity” of aneurysm occlusion devices (32). Many approaches have been presented, ranging from collagen coating or collagen filaments added to platinum coils, ion implantation of extracellular matrix proteins onto coils, and growth factor implantation on embolic devices (10, 11, 13, 15). None of these techniques have reached clinical application. Limitations to prior work include lack of a reliable animal model of aneurysms, in which compelling differences in aneurysmal fibrosis can be proved. It is not known which of these technologies is being considered for commercial application at this time.
Notwithstanding the delay in commercialization of “biologically active” devices, recent clinical data have reinforced the need for aneurysm occlusion devices that may surpass the efficacy of the GDC (5–8). Gruber et al reported a series of 30 large or giant aneurysms treated with the GDC (5). In their series, only 12.5% of giant and 31% of very large aneurysms were totally occluded in the first embolization session, and a large majority of treated aneurysms demonstrated aneurysm regrowth or coil compaction. Their clinical experience has led these authors to recommend that the GDC not be used to treat large and giant aneurysms. These results are not surprising given the growing human histologic database suggesting that the thrombus induced in large and giant aneurysms by GDC embolization remains unorganized for prolonged periods (7, 8). This unorganized thrombus is unable to prevent aneurysm regrowth and coil compaction. Biological modification of coils may allow rapid and complete organization of thrombus in the aneurysm, leading to permanent cure.
Even though most authors have focused on discussions of large and giant aneurysms when arguing for biologically modified coils, there are emerging data that all aneurysms may benefit from such modifications. Cognard et al (6) reported a series of 148 aneurysms, mostly less than 10 mm in diameter, in which 14% of cases demonstrated aneurysm regrowth. This high rate of relatively early aneurysm regrowth even in small aneurysms suggests that biologically modified coils may one day be used to treat all aneurysms, regardless of size.
The pathophysiological mechanisms underlying the lack of stable scar formation in coil-embolized aneurysms remain poorly understood. The process of clot maturation has been extensively defined in animal models. Initially, monocytes present within the thrombus differentiate into myofibroblasts. These myofibroblasts stabilize the thrombus by the production of type I collagen (33, 34). Capillary in-growth from the periphery results in the formation of a vascularized fibrous connective tissue scar. Because platinum microcoils are biologically inert, it is possible that continuous turnover of thrombus within embolized aneurysms precludes differentiation of monocytes into myofibroblasts.
The conclusions and implications of the current study are limited by several technical factors. The process of methylmethacrylate embedding needed for adequate coil histologic analysis inactivates most standard immunohistochemical labeling methods, including the fluorescence membrane dye technique used in this project. As such, we chose to study fibroblast implant viability and proliferation in isolated segments of rabbit carotid artery. In this way, we were able to obtain frozen-section histologic specimens that preserved the fluorescent label of the cells. Work is currently underway to develop alternative labeling techniques that can be used in conjunction with platinum coil embedding and processing procedures.
Preliminary investigations at our institution have suggested that the process of electrolytic detachment used in the GDC system kills or damages cells grown on the coil surface. As a result of this, we chose to sever the cell-coated GDC coils from their pusher wires prior to implantation and deposit them mechanically by using a pusher wire, as is done for nonretrievable coils. The lack of ability to retrieve and reposition these coils would clearly be a disadvantage in the treatment of human aneurysms; however, it is hoped that, with the development of alternative, nonelectrolytic detachment mechanisms currently under development, this will cease to be an issue.
Our data indicate that the delivery of fibroblasts into experimental aneurysms is possible and results in increased fibrosis relative to control findings at 2 weeks post treatment. Whether the early increase in fibrosis will result in significant long-term improvement in rates of aneurysm occlusion and regrowth will be addressed in a future chronic animal study. It is conceivable that the implantation of fibroblast-bearing coils merely accelerates a process that would have occurred eventually with control coils. Previously published work on our experimental aneurysms treated with standard GDC coils, however, documented a relative lack of fibrous tissue in aneurysms up to 12 weeks post treatment, suggesting the early development of fibrosis may indeed result in a long-term benefit (20).
The cells used in this study were immortalized rabbit synovial fibroblasts. As such, there is potential for unconstrained neoplastic proliferation of the cells. Although none of our subjects demonstrated adverse events to suggest this, exclusion of neoplastic activity would require long-term follow-up and extensive histologic evaluation of associated organs such as the brain. Refinement of technique with the use of non-immortalized cells and cells that contain “suicide genes” is currently underway.
The fate of cells lost to the general circulation during delivery is also unknown. By exposing the cell-bearing coils to sustained arterial flow for up to 40 minutes, we were attempting to mimic the potential clinical condition in which a coil may be placed in an aneurysm and then repositioned several times. It is possible, however, that repositioning of coils within an aneurysm may result in cell loss greater than that seen in our model. Additional studies are planned to address in a quantitative fashion the amount of cells lost during delivery and to refine delivery techniques to minimize cell loss.
The issue of potential tissue rejection is a concern if allogenic transplants are to be used. Commercially available skin grafts containing fibroblast allografts are being used clinically, and rejection has as of yet not been a significant problem. The effect of even mild cell rejection on the environment of a treated cerebral aneurysm, however, is unknown at this time and needs further evaluation. Perhaps a mild inflammatory response would have a beneficial effect on aneurysm fibrosis and serve to limit prolonged implanted cell proliferation. Alternatively, a more robust inflammatory response could result in potential destabilization of the aneurysm wall. It is hoped that, because many giant aneurysms are treated in an elective manner, autologous cell lines could be delivered.
The results of this study may have important implications as a potential means of improving treatment of devastating lesions such as giant cerebral aneurysms that are refractory to current modes of therapy. In addition, the technique of targeted endovascular cell delivery could have more widespread application as a new interventional radiology procedure. Medicine is entering new era of biomedical engineering and gene therapy. As physicians trained in endovascular techniques, we are uniquely suited and capable of delivering these genetically based therapies. It is hoped that endovascular cell transplantation may, as technology advances, play a role in treatment of organ-based abnormalities by means of organ regeneration and delivery of genetically based therapies, as well as primary vascular disorders.
Conclusion
Biologically modified embolic coils can be used to deliver fibroblasts directly into experimental aneurysms successfully. Endovascular delivery of fibroblast tissue allografts may provide a means of increasing the biological activity of embolic coils, with the eventual goal of promoting permanent fibrosis of cerebral aneurysms.
TABLE: Pericoil Histologic Grading
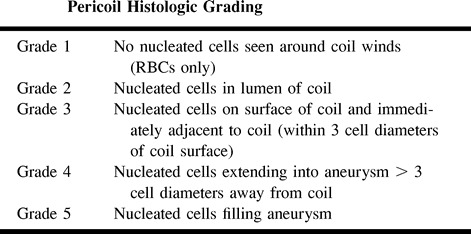
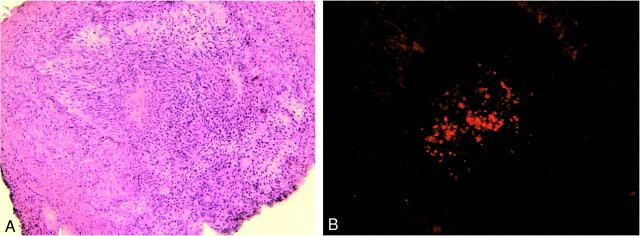
fig 8.
H&E (A) and fluorescent micrograph (B) of artery segment implanted 2 weeks previously with fluorescently labeled cells. The lumen of the arterial segment is nearly completely occluded, with a dense cellular infiltrate. The fluorescent images confirm the presence of labeled cells that are most intensely labeled in the central lumen of the vessel. Weak fluorescent labeling of the cells peripherally is likely secondary to the dilution effect on the membrane dye owing to cell division
fig 11.
Histologic grade immediately adjacent to the implanted coils on the basis of the presence and amount of nucleated fibroblasts. Fibroblasts were seen adjacent to or within the central lumen of coils in eight (88%) of nine anuerysms treated with cell-bearing GDCs. Nucleated cells were present in none of the nine control coil subjects
Footnotes
This study was supported by an ASNR/Berlex Basic Science Fellowship Grant.
Address correspondence to William F. Marx, M.D., Department of Radiology Box 170, University of Virginia Health Sciences Center, Charlottesville, VA 22908.
References
- 1.Gobin YP, Vinuela F, Gurian JH, et al. Treatment of large and giant fusiform intracranial aneurysms with Guglielmi detachable coils. J Neurosurg 1996;84:55-62 [DOI] [PubMed] [Google Scholar]
- 2.Graves VB, Strother CM, Duff TA, Perl J. Early treatment of ruptured aneurysms with Guglielmi detachable coils: effect on subsequent bleeding. Neurosurgery 1995;37:640-647 [DOI] [PubMed] [Google Scholar]
- 3.Guglielmi G, Vinuela F. Intracranial aneurysms. Guglielmi electrothrombotic coils. Neurosurg Clin North Am 1994;5:427-435 [PubMed] [Google Scholar]
- 4.Malisch TW, Guglielmi G, Vinuela F, et al. Intracranial aneurysms treated with the Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg 1997;87:176-183 published erratum appears in J Neurosurg 1998 Feb;88; 359 [DOI] [PubMed] [Google Scholar]
- 5.Gruber A, Killer M, Bavinzski G, Richling B. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7-year, single-center experience. Neurosurgery 1999;45:793-803 [DOI] [PubMed] [Google Scholar]
- 6.Cognard C, Weill A, Spelle L, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology 1999;212:348-356 [DOI] [PubMed] [Google Scholar]
- 7.Bavinzski G, Talazoglu V, Killer M, et al. Gross and microscopic histopathological findings in aneurysms of the human brain treated with Guglielmi detachable coils. J Neurosurg 1999;91:284-293 [DOI] [PubMed] [Google Scholar]
- 8.Molyneux AJ, Ellison DW, Morris J, Byrne JV. Histological findings in giant aneurysms treated with Guglielmi detachable coils. Report of two cases with autopsy correlation [see comments]. J Neurosurg 1995;83:129-132 [DOI] [PubMed] [Google Scholar]
- 9.Szikora I, Wakhloo AK, Guterman LR, et al. Initial experience with collagen-filled Guglielmi detachable coils for endovascular treatment of experimental aneurysms. AJNR Am J Neuroradiol 1997;18:667-672 [PMC free article] [PubMed] [Google Scholar]
- 10.Ahuja AA, Hergenrother RW, Strother CM, Rappe AA, Cooper SL, Graves VB. Platinum coil coatings to increase thrombogenicity: a preliminary study in rabbits [see comments]. AJNR Am J Neuroradiol 1993;14:794-798 [PMC free article] [PubMed] [Google Scholar]
- 11.Dawson RC, Krisht AF, Barrow DL, Joseph GJ, Shengelaia GG, Bonner G. Treatment of experimental aneurysms using collagen-coated microcoils. Neurosurgery 1995;36:133-139 [DOI] [PubMed] [Google Scholar]
- 12.Dawson RC, Shengelaia GG, Krisht AF, Bonner GD. Histologic effects of collagen-filled interlocking detachable coils in the ablation of experimental aneurysms in swine. AJNR Am J Neuroradiol 1996;17:853-858 [PMC free article] [PubMed] [Google Scholar]
- 13.Kallmes DF, Williams AD, Cloft HJ, Lopes MB, Hankins GR, Helm GA. Platinum coil-mediated implantation of growth factor-secreting endovascular tissue grafts: an in vivo study. Radiology 1998;207:519-523 [DOI] [PubMed] [Google Scholar]
- 14.Kallmes DF, Borland MK, Cloft HJ, et al. In vitro proliferation and adhesion of basic fibroblast growth factor-producing fibroblasts on platinum coils. Radiology 1998;206:237-243 [DOI] [PubMed] [Google Scholar]
- 15.Murayama Y, Vinuela F, Suzuki Y, et al. Ion implantation and protein coating of detachable coils for endovascular treatment of cerebral aneurysms: concepts and preliminary results in swine models. Neurosurgery 1997;40:1233-1243 [DOI] [PubMed] [Google Scholar]
- 16.Wallace PK, Palmer LD, Perry-Lalley D, et al. Mechanisms of adoptive immunotherapy: improved methods for in vivo tracking of tumor-infiltrating lymphocytes and lymphokine-activated killer cells. Cancer Res 1993;53:2358-2367 [PubMed] [Google Scholar]
- 17.Horan PK, Melnicoff MJ, Jensen BD, Slezak SE. Fluorescent cell labeling for in vivo and in vitro cell tracking. Methods Cell Biol 1990;33:469-490 [DOI] [PubMed] [Google Scholar]
- 18.Slezak SE, Horan PK. Fluorescent in vivo tracking of hematopoietic cells. Part I. Technical considerations. Blood 1989;74:2172-2127 [PubMed] [Google Scholar]
- 19.Cloft HJ, Altes TA, Marx WF, et al. Endovascular method of creating experimental aneurysms in rabbits. Radiology 1999;213:223-228 [DOI] [PubMed] [Google Scholar]
- 20.Kallmes DF, Helm GA, Altes TA, Do HM, Mandell JW, Cloft HJ. Histologic evaluation of platinum coil embolization of a novel aneurysm model in rabbits. Radiology 1999;213:217-222 [DOI] [PubMed] [Google Scholar]
- 21.Abruzzo T, Shengelaia GG, Dawson RC, Owens DS, Cawley CM, Gravanis MB. Histologic and morphologic comparison of experimental aneurysms with human intracranial aneurysms. AJNR Am J Neuroradiol 1998;19:1309-1314 [PMC free article] [PubMed] [Google Scholar]
- 22.Raymond J, Desfaits AC, Roy D. Fibrinogen and vascular smooth muscle cell grafts promote healing of experimental aneurysms treated by embolization. Stroke 1999;30:1657-1664 [DOI] [PubMed] [Google Scholar]
- 23.Naughton GK. Lanza RP LRCW. Skin and epithelia. Principles of Tissue Engineering 1st ed. San Diego: Academic Press 1997;769-781
- 24.Liu Y, Himes BT, Tryon B, et al. Intraspinal grafting of fibroblasts genetically modified by recombinant adenoviruses. Neuroreport 1998;9:1075-1079 [DOI] [PubMed] [Google Scholar]
- 25.Jones GE. Cultures of proliferating vascular smooth muscle cells. In: Kirschenlohr HL, Metcalfe JC, Grainger DJ, et al, eds. Methods in Molecular Medicine: Human Cell Culture Protocols Totowa, NJ: Humana Press Inc. 1996;319-334 [DOI] [PubMed]
- 26.Theobald VA, Lauer JD, Kaplan FA, Baker KB, Rosenberg M. “Neutral allografts”—lack of allogeneic stimulation by cultured human cells expressing MHC class I and class II antigens. Transplantation 1993;55:128-133 [DOI] [PubMed] [Google Scholar]
- 27.Eaglstein WH, Falanga V. Tissue engineering and the development of Apligraf, a human skin equivalent. Clin Ther 1997;19:894-905 [DOI] [PubMed] [Google Scholar]
- 28.Parenteau N. Skin: the first tissue-engineered products. Sci Am 1999;280:83-84 [DOI] [PubMed] [Google Scholar]
- 29.Eaglstein WH, Alvarez OM, Auletta M, et al. Acute excisional wounds treated with a tissue-engineered skin (Apligraf). Dermatol Surg 1999;25:195-201 [DOI] [PubMed] [Google Scholar]
- 30.Dolynchuk K, Hull P, Guenther L, et al. The role of Apligraf in the treatment of venous leg ulcers. Ostomy Wound Manage 1999;45:34-43 [PubMed] [Google Scholar]
- 31.Sibbald RG. Apligraf living skin equivalent for healing venous and chronic wounds. J Cutan Med Surg 1998;3:S1-S8 [PubMed] [Google Scholar]
- 32.Nichols DA. Endovascular therapy as the preferred method of treatment for “surgical” aneurysms: what must happen for this to become reality? AJNR Am J Neuroradiol 1999;20:1391-1392 [PMC free article] [PubMed] [Google Scholar]
- 33.Feigl W, Susani M, Ulrich W, Matejka M, Losert U, Sinzinger H. Organisation of experimental thrombosis by blood cells. Evidence of the transformation of mononuclear cells into myofibroblasts and endothelial cells. Virchows Archiv. A, Pathol Anat Histopathol 1985;406:133-148 [DOI] [PubMed] [Google Scholar]
- 34.Leu HJ, Feigl W, Susani M, Odermatt B. Differentiation of mononuclear blood cells into macrophages, fibroblasts and endothelial cells in thrombus organization. Exper Cell Biol 1988;56:201-210 [DOI] [PubMed] [Google Scholar]