Abstract
BACKGROUND AND PURPOSE: MR imaging has been shown to be of prognostic significance in the evaluation of asphyxiated neonates. The purpose of this project was to determine whether the use of intensity ratios in key regions of the brain might better detect regions of injured brain and thus improve the correlation of imaging findings with 12-month neurodevelopmental outcome.
METHODS: Prospectively acquired MR studies of 53 asphyxiated neonates were reviewed retrospectively. Signal intensities from standard T1- and T2-weighted images of seven major brain regions that are affected in asphyxia were measured. Intensity ratios were calculated by dividing the signal intensity of each brain region by the signal intensity of the ocular vitreous. The intensity ratios were then correlated with 12-month neurodevelopmental outcome. These results were compared with correlations determined by a qualitative scoring system.
RESULTS: The only significant statistical correlation between the intensity ratios and 12-month neurodevelopmental outcome were those of anterior watershed injury with the Mental Development Index of the Bayley Scales of Infant Development II. The qualitative measurements showed a strong correlation with many outcome parameters.
CONCLUSION: Standard qualitative assessment is more predictive of neurodevelopmental outcome than is quantitative analysis. This finding most likely reflects the inability of the quantitative assessment of intensity ratios to compensate for the day-to-day evolution of signal intensity of the injured neonatal brain. Anterior watershed injury may be predictive of abnormal cognitive outcome; examination of these patients at age 30 months will be important to determine the accuracy of this observation.
Hypoxic-ischemic encephalopathy is one of the major causes of morbidity in newborns (1, 2). Several studies in humans and animals have shown that early detection of CNS injuries is possible using various MR imaging techniques (3–9). The changes seen on routine T1- and T2-weighted sequences are characteristic, but are often subtle and may not be apparent until several days after the injury (6, 7, 9, 10). Early detection is important, because it may allow early intervention to prevent further deterioration of the CNS insult through the use of special neuroprotective agents. Furthermore, it is crucial to know if the brain of an imaged neonate is normal in order to avoid potential undesirable side effects of neuroprotective agents on a healthy brain (7, 11).
The use of standard imaging sequences in asphyxiated neonates is limited by several factors: 1) standard sequences are typically evaluated qualitatively and these qualitative evaluations are difficult to reproduce; 2) diffuse injuries may be difficult to detect if all parts of the brain are equally affected; and 3) the qualitative nature of standard sequences makes them difficult to compare with the easily quantitated diffusion images.
In this project, intensity ratios were calculated from regions of interest (ROIs) on standard MR sequences of asphyxiated neonates to test whether quantitation might improve the reproducibility of image evaluation and increase sensitivity of standard imaging techniques for detecting CNS injuries in the acute phase. We then correlated the intensity ratios with outcome to determine whether there were significant differences in intensity measurements between those with normal and abnormal neurodevelopmental outcome at age 12 months. We also investigated which MR sequence has the best correlation with 12-month neuromotor examination and mental development. Finally, we compared the results with those of a qualitative scoring system, which was assessed on standard MR sequences.
Methods
Patient Data
At the time of this study, 4082 consecutive term babies admitted to our neonatal intensive care unit had been screened prospectively as part of an ongoing study investigating the utility of MR imaging in assessing brain injury of asphyxiated neonates. Entry criteria for the study were 1) umbilical artery pH less than 7.1, 2) umbilical artery bases deficit greater than 10, or 3) 5-minute Apgar score less than or equal to 5. Infants with suspected or confirmed congenital malformations or congenital infections and those born before 36 weeks' gestational age (in total, 71 infants) were excluded from the study. The protocol was approved by the committee on human research at our institution. Participation in the study was voluntary; the babies were studied only after informed consent was obtained from their parents. Of the 110 babies that met the inclusion criteria, 15 declined (via their parents) to be studied by MR imaging after initially enrolling in the study. Thus, a total of 95 babies were enrolled and studied, and, of those, 53 (33 boys and 20 girls) who underwent 12-month neuromotor and mental development examinations are reported in this article.
MR Data
Attempts were made to perform the MR study as soon as consent was obtained from the parents and the neonate was considered stable enough to be transported to the MR unit. The MR examination, performed at 1.5 T using the standard quadrature head coil with no aids (such as water bags), consisted of noncontrast 4-mm axial spin-echo (500/11/2 [TR/TE/excitations]) images and 4-mm axial spin-echo (3000/60,120/1) images through the entire brain. Both quantitative and qualitative scoring was performed for all three of these sequences for each patient. The quantitative measurements were performed by placing ROI boxes (each measuring 40 mm2) in seven standardized places: the basal ganglia, thalamus, anterior frontal white matter, anterior and posterior watershed areas, and pre- and postrolandic regions (See Table 1 and Fig 1). These seven voxel locations were determined on the basis of reports showing that these are the major regions that are affected in asphyxiated neonates (1, 2, 12, 13). For every region, a standardized voxel measurement was obtained (Table 1). Because of variations in intensity due to coil loading and other acquisition conditions, all measurements were expressed as ratios with the intensity of the regional ROI as the numerator and the intensity of the vitreous of the ocular globe on the same sequence as the denominator.
TABLE 1:
The standardized locations of regions of interest
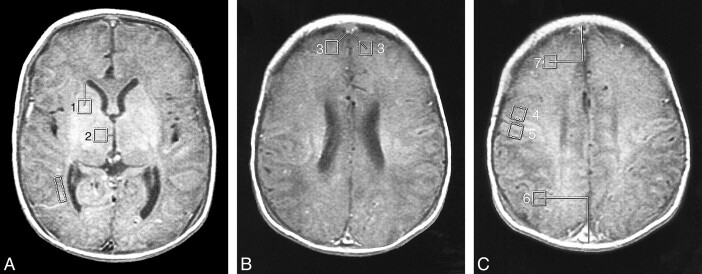
fig 1.
Locations of the 40-mm2 voxels used in the study (see Table 1). The location of the eyeball voxel is obvious and not illustrated. The lines show how the measurements were made to keep the locations consistent.
A, MR image shows location of voxels for basal ganglia (1) and thalamus (2). (The box adjacent to the trigone/occipital horn of the right lateral ventricle was for another project.)
B, MR image shows location of voxels for frontal white matter (3).
C, MR image shows location of voxels for prerolandic (4), postrolandic (5), posterior watershed (6), and anterior watershed (7) areas.
All intensity measurements were performed by two radiologists, who were blinded to the qualitative scores and to all clinical parameters and outcomes. Measurements in the first 10 patients were performed twice by both radiologists. Analysis of the data revealed that both the inter- and intraobserver variability of the ratio measurements was less than 2%. Subsequent measurements were performed by one radiologist for all patients.
The qualitative assessments and scoring were performed as described in the literature (6). As in that analysis, three scores were assigned: basal ganglia, watershed (W), and combined basal ganglia/watershed. These assessments were performed twice, at different times, in each patient by a single neuroradiologist, who was blinded to the quantitative scores and the clinical parameters and outcomes. Intraobserver variation for the qualitative scores was 1.5%. Some of these scores have been reported previously (6).
Neurodevelopmental Examinations
All 53 subjects underwent a neurologic examination at age 12 months by an experienced child neurologist, who was blinded to the results of the imaging studies and to the clinical course of the infant. The results of the neurologic examination were classified as normal or abnormal. The five infants who died before undergoing neuromotor examination at 12 months all had significant neurologic abnormalities detected before death and were included in the study (and classified as abnormal). In addition to the standard neurologic examination, development was assessed by administering the Mental Developmental Index (MDI) of the Bayley Scales of Infant Development II (14).
Data Analysis
Univariate assessments of association of the various MR ratios with outcome were performed in a variety of ways, depending on outcome type. For the binary (normal/abnormal) classification of neurologic examination at 12 months, the MR ratios were compared between normal and abnormal groups using the t test and Wilcoxon two-sample test. Boxplots were used to visualize differences and appraise test assumptions; no indications of lack of symmetry emerged, so transformations were not pursued. For the continuous MDI at 12 months, univariate linear regressions were performed for each MR ratio. Corresponding scatterplot smooths of MDI against each MR ratio were used to assess linearity. Multivariate analyses included stepwise and subset selection strategies to fit logistic (for neurologic examination) and multiple (for MDI) regression models. A parallel analysis plan was used to evaluate the qualitative variables. Qualitative data were correlated with outcome parameters as described previously (6).
Results
Quantitative Analysis
The statistical analysis (Table 2) showed essentially no significant association between quantitative MR measurements and 12-month neuromotor outcome. The following measurements were significantly different between the normal and abnormal 12-month MDI assessments: frontal white matter, T1-weighted images (P = .005); thalamus, first-echo T2-weighted images (P = .02); and thalamus, second-echo T2-weighted images (P = .02). Only the T1 anterior watershed measurement remained significant (P = .0005) in the multivariate linear model, most likely reflecting strong collinearity among the variables (eg, the correlation between regression coefficients for T2 thalamus measurements and proton-density basal ganglia was −0.8). There was no significant difference between the sequences in the quantitative assessment.
TABLE 2:
Quantitative analysis A: Results from stepwise logistic regression using quantitative MR variables
Qualitative Analysis
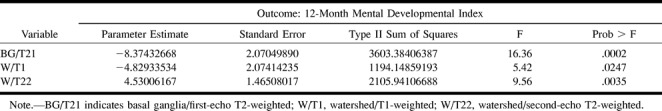
In contrast to the quantitative measurements, there was a very strong association between qualitative assessment and developmental and motor outcome (Table 3). Associations between the MR score and 12-month neuromotor outcome showed the following scores to be significantly different between normal and abnormal outcome groups: basal ganglia, T1-weighted images (P = .005); basal ganglia, first-echo T2-weighted images (P < 0.0001); basal ganglia, second-echo T2-weighted images (P < .0001); basal ganglia/watershed, T1-weighted images (P = .002); basal ganglia/watershed, first-echo T2-weighted images (P = .01); and basal ganglia/watershed, second-echo T2-weighted images (P = .01). The following MR scores showed significant associations with MDI: basal ganglia, first-echo T2-weighted images (P = .002); basal ganglia, second-echo T2-weighted images (P = .005); watershed, T1-weighted images (P = .02); and basal ganglia/watershed, T1-weighted images (P = .002).
TABLE 3:
Qualitative Analysis A: Results from stepwise logistic regression using qualitative MR variables
Discussion
The results of our study indicate that quantitative intensity measurements of T1- and T2-weighted MR images obtained during the first 10 days of life in neonates meeting the criteria for our study essentially showed a correlation only of the frontal watershed region with 12-month cognitive development. In contrast, good correlations were found between qualitative assessment and those same outcomes. This result is somewhat unexpected, as our pretest hypothesis was that the quantitative assessment should be more sensitive to small changes in signal intensity that may not be detectable to the eye of the observer.
A number of possible explanations can be suggested for the better correlation of the qualitative than the quantitative assessment. The most obvious stems from the fact that the imaging characteristics of the injured neonatal brain are complex. The regions that are injured vary with the type of injury (mild-to-moderate hypotension versus profound hypotension), severity of injury, and postconceptional age of the infant at the time of injury (12, 15, 16). Depending on these factors, the injury might involve the entire cerebral cortex, intervascular boundary zones, perirolandic region, basal ganglia, or brain stem. We attempted to take these variations into account by measuring all these regions. However, intervascular boundary zones vary with vascular anatomy, and it is well known that vascular anatomy varies considerably among patients. Therefore, it is entirely possible that our voxel locations may have partially or completely missed the regions in which the injuries occurred. This may have been remedied by using larger voxels. However, large voxels can give false-negative results when the area of injury is small; therefore, the change in image intensity can be obscured by averaging with a large percentage of normal tissue within the ROI. Perhaps the qualitative grading system is more accurate, because it allows the interpreter to view a more general area in search of signal abnormalities.
Another potential source of error in the analysis of the quantitative study stems from the fact that the signal intensity of the injured tissue varies with the amount of time that has elapsed between injury and imaging examination (10, 12, 15). Initially, the injured region of the brain shows T1 and T2 prolongation. Then, at 2 to 3 days, T1 shortening develops. T2 shortening may develop by day 6 (10). Thus, we were faced with the problem that the injured tissue may appear normal, hypointense, or hyperintense depending on when the imaging study was performed. We tried to compensate for this temporal variation in our analysis by grouping patients of similar age and by looking at absolute deviations of intensity measurements from the norm (ignoring whether the abnormality showed hyper- or hypointense signal), but this did not improve the statistical results. We suspect that the results of the quantitative analysis could be improved if all the neonates had been scanned at the same hour or day after the injury. A number of factors prevented us from achieving such uniform timing in the MR studies. The most obvious of these was that the precise time of the injury was not always known and could not be controlled. Other factors are possible, but often difficult, to control. For example, because this was a research study, it was necessary to obtain parental consent before scheduling the MR examination; members of the study team were not always available (on weekends, for example) to explain the study to the parents, often resulting in a delay of several days before consent was obtained. Once consent was obtained, it was sometimes difficult to get an appointment for our busy inpatient MR scanner. Patients who were very ill and in need of an imaging study for clinical management decisions are usually imaged within 24 hours of the time of request. However, some of our neonates were not particularly sick and had much lower priority for getting on the schedule; indeed, some of our infants recovered quickly from their difficulties during the birth process, were discharged, and returned to have their MR studies as outpatients. In addition, there was an impression early in the course of our study that imaging was less helpful if performed before the age of 3 days, so the imaging of some neonates was intentionally delayed. The variation in the timing of the scans was less a problem in interpreting the qualitative studies, as the regions were judged only as normal or abnormal, so hypo- versus hyperintense signal was not a significant issue.
Another potential problem stemmed from the use of signal intensity as a variable. It is well known that signal intensity measurements on spin-echo MR studies are dependent on many factors, including proton density, T1 and T2 relaxation times, and scaling (17). No universally agreed upon standards have been established for measuring and reporting these intensity values. As our study intentionally replicates the sequences used in normal clinical studies, we had only single-echo T1-weighted and dual-echo T2-weighted images; thus, it was not possible to calculate accurate T1 and T2 relaxation times from our data. To compensate for the scaling by the MR scanner, we converted our intensity measurements (obtained by sampling our ROIs on a GE Advantage Windows workstation) into intensity ratios using the intensity of the vitreous of the ocular globe as the denominator. By using the vitreous (which should not change in intensity as a result of asphyxia) as the denominator, the effects of windowing and scaling should be negated. Thus, we do not believe that our use of intensity measurements was responsible for the lack of correlation between quantitative measurements and outcome.
The question arises as to whether the use of newer MR techniques that can be more precisely quantified, such as diffusion imaging and spectroscopy, might eliminate some of the difficulties that we encountered in this study. Although the numerical data obtained by MR diffusion imaging and spectroscopy might allow easier physical interpretation, many of the complexities encountered in this study would remain. Both diffusion values and metabolite ratios change with time after a hypoxic-ischemic incident. Diffusion values are initially reduced relative to normal values, but increase and reach normal levels at 5 to 10 days before increasing further as a result of breakdown of the cells (18, 19). In addition, it has become apparent that diffusion images may be transiently normal during the first day of life, presumably after normalization of initial mitochondrial dysfunction, but before secondary energy failure has ensued (20, 21). Metabolic ratios as measured by MR spectroscopy, too, can vary in the first few days after neonatal injury (4, 22–24). Thus, it seems that even with more sophisticated techniques, difficulties are likely to arise as a result of problems with consistent timing of the studies. More sophisticated statistical methods (eg, tree-structured techniques, neural nets, multiple additive regression splines) might elicit more predictive functional forms or combinations of the MR ratio variables.
Nonetheless, after having rationalized why we obtained fewer correlations by the quantitative measurements, the one correlation that was significant on multivariate analysis, the correlation of quantitative frontal watershed injury with 12-month MDI, is deserving of some discussion. The fact that the quantitative analysis showed correlation with MDI but not with neuromotor outcome correlates well with what we know about the prefrontal regions: that they are important in cognition but not in motor activities. Indeed, the prefrontal regions are the most recently developed areas of the brain from a phylogenetic and ontogenetic perspective (25). This observation suggests that the quantitative analysis may be less sensitive to the detection of motor injury but equally sensitive to injury in some regions that relate to nonmotor neurologic outcome. This is potentially a very important finding, suggesting that accurate assessment of the prefrontal regions might be able to predict abnormalities in higher cortical functioning. Correlation with 30-month follow-up examinations, which are now in progress, may help to further elucidate this potentially important observation.
Conclusion
Our study suggests that qualitative visual assessment of standard spin-echo MR studies of neonates at risk for hypoxic-ischemic encephalopathy is more predictive of outcome than quantitative assessment of standardized locations at risk for involvement. It remains to be seen whether the use of new, more easily quantifiable techniques, such as diffusion imaging and spectroscopy, will provide more accurate quantitative assessments of neonatal asphyxic brain injury. Our results also suggest that anterior watershed injury may be predictive of abnormal cognitive outcome; examination of these patients at age 30 months will be important to determine the accuracy of this observation.
TABLE 2:
B: Results from stepwise linear regression using quantitative MR variables

TABLE 2:
C: Results from stepwise linear regression using quantitative MR variables
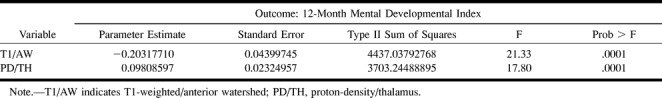
TABLE 3:
B: Results from stepwise linear regression using qualitative MR variables
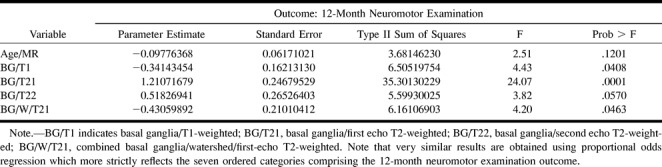
TABLE 3:
C: Results from stepwise linear regression using qualitative MR variables
Footnotes
Supported by NIH grant NS35902 and NIH Clinical Research Center Grant MO1RR01271.
Address reprint requests to A. James Barkovich, MD, Neuroradiology Section, Room L-371, UCSF, 505 Parnassus Ave, San Francisco, CA 94143.
References
- 1.Volpe JJ. Hypoxic-ischemic encephalopathy: neuropathology and pathogenesis. In: Volpe JJ, ed. Neurology of the Newborn. 3rd ed. Philadelphia: Saunders 1995:279-313 [Google Scholar]
- 2.Barkovich AJ. MR and CT evaluation of profound neonatal and infantile asphyxia. AJNR Am J Neuroradiol 1992;13:959-972 [PMC free article] [PubMed] [Google Scholar]
- 3.Hanrahan JD, Cox IJ, Azzopardi D, et al. Relation between proton magnetic resonance spectroscopy within 18 hours of birth asphyxia and neurodevelopment at 1 year of age. Dev Med Child Neurol 1999;41:76-82 [DOI] [PubMed] [Google Scholar]
- 4.Barkovich AJ, Baranski K, Vigneron D, et al. Proton MR spectroscopy in the evaluation of asphyxiated term neonates. AJNR Am J Neuroradiol 1999;20:1399-1405 [PMC free article] [PubMed] [Google Scholar]
- 5.Amess PN, Penrice J, Wylezinska M, et al. Early brain proton magnetic resonance spectroscopy and neonatal neurology related to neurodevelopmental outcome at 1 year in term infants after presumed hypoxic-ischaemic brain injury. Dev Med Child Neurol 1999;41:436-445 [PubMed] [Google Scholar]
- 6.Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR Am J Neuroradiol 1998;19:143-150 [PMC free article] [PubMed] [Google Scholar]
- 7.D'Arcueil H, Crespigny A, Rother J, et al. Diffusion and perfusion magnetic resonance imaging of the evolution of hypoxic ischemic encephalopathy in the neonatal rabbit. J Magn Reson Imaging 1998;8:820-828 [DOI] [PubMed] [Google Scholar]
- 8.Kuenzle C, Baenziger O, Martin E, et al. Prognostic value of early MR imaging in term infants with severe perinatal asphyxia. Neuropediatrics 1994;25:191-200 [DOI] [PubMed] [Google Scholar]
- 9.Rutherford M, Pennock J, Schwieso J, et al. Hypoxic ischaemic encephalopathy: early and late magnetic resonance findings in relation to outcome. Arch Dis Child 1996;75:145-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkovich AJ, Westmark KD, Ferriero DM, Sola A, Partridge JC. Perinatal asphyxia: MR findings in the first 10 days. AJNR Am J Neuroradiol 1995;16:427-438 [PMC free article] [PubMed] [Google Scholar]
- 11.Vannucci RC, Perlman JM. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics 1997;100:1004-1014 [DOI] [PubMed] [Google Scholar]
- 12.Barkovich AJ. Brain and spine injuries in infancy and childhood. In: Barkovich AJ, ed. Pediatric Neuroimaging. 3rd ed. Philadelphia: Lippincott Williams & Wilkins 2000:157-249 [Google Scholar]
- 13.Barkovich AJ, Hallam D. Neuroimaging in perinatal hypoxic-ischemic injury. MRDD Res Rev 1997;3:28-41 [Google Scholar]
- 14.Bayley N. The Bayley Scales of Infant Development II.. New York: New York Psychological Corp; 1993
- 15.Barkovich AJ, Sargent SK. Profound asphyxia in the preterm infant: imaging findings. AJNR Am J Neuroradiol 1995;16:1837-1846 [PMC free article] [PubMed] [Google Scholar]
- 16.Barkovich AJ, Truwit CL. MR of perinatal asphyxia: correlation of gestational age with pattern of damage. AJNR Am J Neuroradiol 1990;11:1087-1096 [PMC free article] [PubMed] [Google Scholar]
- 17.Cox I, Roberts T, Moseley M. Principles and techniques in neuroimaging. In: Kucharczyk J, Moseley M, Barkovich AJ, eds. Magnetic Resonance Neuroimaging. Boca Raton, FL: CRC 1994:1-102 [Google Scholar]
- 18.Cowan FM, Pennock JM, Hanrahan JD, Manji KP, Edwards AD. Early detection of cerebral infarction and hypoxic ischemic encephalopathy in neonates using diffusion weighted magnetic resonance imaging. Neuropediatrics 1994;25:172-175 [DOI] [PubMed] [Google Scholar]
- 19.Burdette J, Ricci P, Petitti N, Elster A. Cerebral infarction: time course of signal intensity changes on diffusion-weighted MR images. AJR Am J Roentgenol 1998;171:791-795 [DOI] [PubMed] [Google Scholar]
- 20.Robertson R, Ben-Sira L, Barnes P, et al. MR line scan diffusion weighted imaging of term neonates with perinatal brain ischemia. AJNR Am J Neuroradiol 1999;20:1658-1670 [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton JS, Ordidge RJ, Penrice J, et al. Temporal and anatomical variations of brain water apparent diffusion coefficient in perinatal cerebral hypoxic-ischemic injury: relationships to cerebral energy metabolism. Magn Reson Med 1998;39:920-927 [DOI] [PubMed] [Google Scholar]
- 22.Penrice J, Lorek A, Cady EB, et al. Proton magnetic resonance spectroscopy of the brain during acute hypoxia-ischemia and delayed cerebral energy failure in the newborn piglet. Pediatr Res 1997;41:795-802 [DOI] [PubMed] [Google Scholar]
- 23.Penrice J, Cady EB, Lorek A, et al. Proton magnetic resonance spectroscopy of the brain in normal preterm and term infants and early changes after perinatal hypoxia-ischemia. Pediatr Res 1996;40:6-14 [DOI] [PubMed] [Google Scholar]
- 24.Hanrahan JD, Sargentoni J, Azzopardi D, et al. Cerebral metabolism within 18 hours of birth asphyxia: a proton magnetic resonance spectroscopy study. Pediatr Res 1996;39:584-590 [DOI] [PubMed] [Google Scholar]
- 25.Kiernan JA. Barr's The Human Nervous System: An Anatomical Viewpoint.. Philadelphia: Lippincott Williams & Wilkins 1998:292-293 [Google Scholar]