Abstract
BACKGROUND AND PURPOSE: Developmental changes in hippocampal formations (HFs) have been reported in association with agenesis of the corpus callosum, lissencephaly, and holoprosencephaly. The purpose of this study was to evaluate the developmental changes in HFs in patients with a variety of other congenital brain malformations.
METHODS: MR images of 44 patients with congenital brain malformations associated with 11 different brain disorders were reviewed retrospectively. Five patients had more than two anomalies. Imaging and clinical findings were evaluated for the shape, size, degree of inversion, and side of abnormal HF.
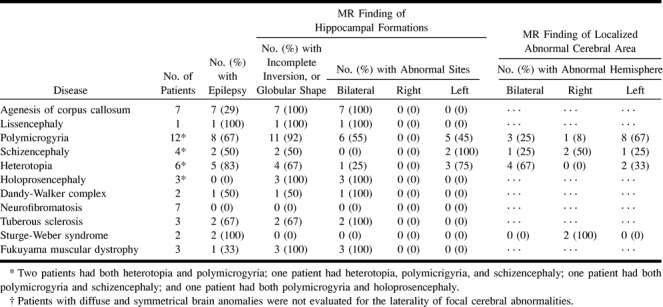
RESULTS: Vertically oriented or globular-shaped HFs were observed in 28 patients (64%) on coronal MR images. All patients with agenesis of the corpus callosum (n = 7), lissencephaly (n = 1), holoprosencephaly (n = 3), and Fukuyama muscular dystrophy (n = 3) had an abnormal HF. A high prevalence of abnormalities was observed in patients with polymicrogyria (11/12, 92%), heterotopia (4/5, 80%), tuberous sclerosis (2/3, 67%), and schizencephaly (2/4, 50%). Patients whose abnormalities were symmetrical had bilateral abnormal HFs, whereas those with polymicrogyria, schizencephaly, and heterotopia, whose abnormalities were localized, tended to have unilateral abnormal HFs.
CONCLUSION: Hippocampal developmental abnormalities are found in a high percentage of patients with congenital malformations. Focusing on the morphologic abnormalities of the HF on coronal MR images may help in the detection of diseases associated with brain anomalies, especially subtle cortical disorders.
The advent of MR imaging has allowed the clear depiction of anatomic structures of the brain and has contributed to the investigation of brain development. Various developmental brain anomalies, such as several types of cortical dysgenesis, can now be diagnosed using MR imaging. For example, mesial temporal sclerosis has been reported to be one of the major causes of temporal lobe epilepsy (1, 2), but the underlying structural abnormality remained undetected in vivo until the emergence of MR imaging.
Hippocampal abnormalities have been observed not only in mesial temporal sclerosis but also in other diseases. Developmental changes in hippocampal formations (HFs) have been reported in agenesis of the corpus callosum, lissencephaly, and holoprosencephaly (3–5). Such investigations have found that HFs were small in 62% of cases and that abnormal vertical orientation was present in 82% of patients (6). These findings seem to be reasonable, because inversion of the HF is one of the expressions of normal gyration. Hippocampal abnormalities may be expected to be observed in association with other cortical developmental disorders; however, no such investigation has been undertaken.
The purpose of this study was to evaluate the developmental changes in HF on MR images obtained in patients with a wide variety of congenital brain malformations (7) and to explore the relationship between disease and epilepsy and between the side of abnormal HF and the laterality of abnormal cerebral areas.
Methods
Patients
MR images of 44 patients with congenital malformations of the brain were reviewed retrospectively. Disorders included neural tube closures (Chiari malformations, cephaloceles, corpus callosum anomalies), diverticulation and cleavage (holoprosencephaly), sulcation and cellular migration (lissencephaly, polymicrogyria, heterotopia, schizencephaly, and unilateral megalocephaly), posterior fossa malformations (Dandy-Walker malformations), and neurocutaneous syndrome (neurofibromatosis, tuberous sclerosis, Sturge-Weber syndrome) (7). Congenital muscular dystrophy (Fukuyama muscular dystrophy, Walker-Warburg syndrome) was also investigated in this study, because some types of this disorder are associated with anomalies of the brain and eyes (8, 9).
We identified 78 patients by medical or radiologic records who were referred to our institutes between January 1993 and September 1999. To evaluate HFs on MR studies, we selected coronal MR images that had been obtained with a slice thickness of less than 5 mm. Thirty-four patients were excluded from the study because they had not undergone MR examination (n = 7) or coronal MR studies were not obtained (n = 24) or the slice thickness was greater than 5 mm (n = 3). No patients with hydrocephalus were included. The remaining 44 patients (24 male and 20 female, ranging in age from 1 month to 50 years; mean age, 11 years) had a variety of diseases that included agenesis of the corpus callosum (n = 7), lissencephaly (n = 1), polymicrogyria (n = 12), schizencephaly (n = 4), heterotopia (n = 6), holoprosencephaly (n = 3), Dandy-Walker complex (n = 2), neurofibromatosis (n = 7), tuberous sclerosis (n = 3), Sturge-Weber syndrome (n = 2), and Fukuyama muscular dystrophy (n = 3) (five patients had more than two anomalies, see Table 1). The presence or absence of seizure disorder was noted for each patient.
TABLE 1:
MR and clinical findings in 44 patients with congenital and developmental malformations of the brain
Imaging Protocol and Evaluation
All MR studies were performed on 1.5-T MR units. Axial T1- and T2-weighted images were routinely obtained with or without sagittal T1-weighted axial or coronal fluid-attenuated inversion-recovery (FLAIR) sequences. Twenty coronal images were obtained with spin-echo (SE) T1-weighted sequences, eight with inversion-recovery (IR) sequences, eight with 3D spoiled gradient-recalled acquisition in the steady state (3D-SPGR) sequences, five with SE T2-weighted sequences, one with both SE T1- and T2-weighted sequences, one with both SE T2- and IR sequences, and one with both 3D-SPGR and FLAIR sequences. For SE T1-weighted sequences, imaging parameters were 400–570/9–18/2–4 (TR/TE/excitations); for SE T2-weighted images, 2000–4000/90–128/1–2; for IR sequences; 2000/16–25/1–2, IR = 900; for 3D-SPGR sequences, 11.2–14.2/4.2–5.5/1, flip angle = 45°; and for FLAIR sequences, 8000–10002/140–162/1, IR = 2000–2200. The matrix was 256 × 165 or 256 × 192 or 256 × 224 or 514 × 384. The field of view ranged from 16 to 22 cm. The slice thickness of the coronal images varied from 3 to 5 mm with a 0- to 2.5-mm intersection gap. Two radiologists evaluated the size, shape, and degree of inversion of the HF and the side of abnormal HFs on coronal MR images as well as the location of abnormal cerebral areas.
Results
The MR and clinical findings of the 44 subjects with congenital malformations of the brain are listed in Table 1. In general, we found no significant correlation between epilepsy and hippocampal abnormality. A high prevalence of epilepsy was noted in patients with lissencephaly, polymicrogyria, schizencephaly, heterotopia, tuberous sclerosis, and Sturge-Weber syndrome. Twenty-eight patients (64%) had an abnormal HF (more vertically oriented, or globular in shape) on MR images. In 22 of these, the HF had an oval shape with a long axis and was vertically oriented; seven were small. In the remaining six, the HF was globular in shape and four of these were small. Fourteen (74%) of 19 patients with epilepsy had an abnormal HF, and 13 (46%) of 28 patients with an abnormal HF had epilepsy. In patients with lissencephaly, polymicrogyria, schizencephaly, heterotopia, or tuberous sclerosis, the rate of both epilepsy and hippocampal abnormality was high, but no such relationship was observed in patients with holoprosencephaly or Sturge-Weber syndrome (Table 1).
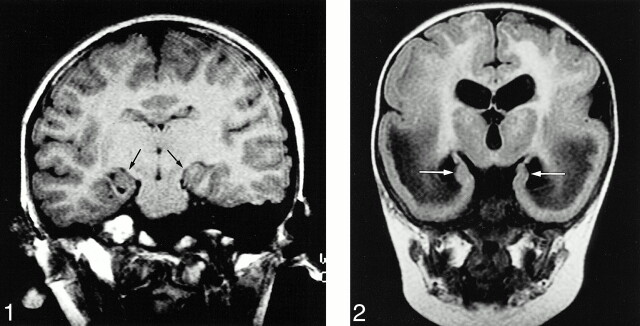
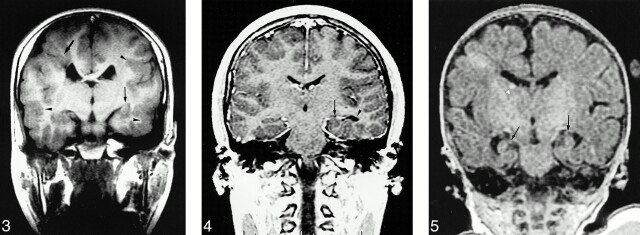
Abnormal HFs were observed in all patients with agenesis of the corpus callosum, lissencephaly, holoprosencephaly (Fig 1), and Fukuyama muscular dystrophy (Fig 2). Eleven (92%) of the 12 patients with polymicrogyria had an abnormal HF (Fig 3), and hippocampal abnormalities were found in four (67%) of the six patients with heterotopia (Fig 4), two (67%) of the three patients with tuberous sclerosis (Fig 5), two (50%) of the four patients with schizencephaly (Fig 3), and one (50%) of the two patients with Dandy-Walker complex. HFs were normal in all patients with neurofibromatosis and Sturge-Weber syndrome.
fig 1.
11-year-old boy with holoprosencephaly. Coronal T1-weighted image (420/15/2) shows bilateral vertically oriented HFs (arrows).fig 2. One-year-old infant with congenital Fukuyama muscular dystrophy. On coronal FLAIR image (10002/162/1, TI = 2200), neither HF shows inversion (arrows), their configurations are straight and vertical. Both temporal lobes have a smooth cortical surface (lissencephaly) with abnormal low-intensity white matter. Both frontal lobes have shallow sulci
fig 3.
Nine-year-old boy with bilateral polymicrogyria and schizencephaly. Coronal T1-weighted image (420/15/2) shows vertical left HF (thin arrow). Bilateral frontotemporal polymicrogyria (arrowheads) and right schizencephaly (thick arrow) are also present.fig 4. 23-year-old woman with left temporooccipital heterotopia. Contrast-enhanced coronal 3D-SPGR image (11.2/4.2/1, flip angle = 45°) shows left hippocampal formation, located medially and globular in shape (arrow), with asymmetrically dilated inferior horn of left lateral ventricle (arrowhead).fig 5. One-month-old baby with tuberous sclerosis. Coronal 3D-SPGR image (14.2/5.5/1, flip angle = 45°) shows both HFs are small and vertical (arrows). Tiny high-intensity subependymal tubers (arrowhead) are also evident
All patients with agenesis of the corpus callosum, holoprosencephaly, Dandy-Walker complex, tuberous sclerosis, and Fukuyama muscular dystrophy, whose abnormalities were diffuse or symmetrical, had bilateral abnormal HFs (Table 1; Figs 1, 2, and 5). On the other hand, patients with polymicrogyria, schizencephaly (Fig 3), and heterotopia (Fig 4), whose abnormalities were localized, had same-sided abnormal HFs (Table 1).
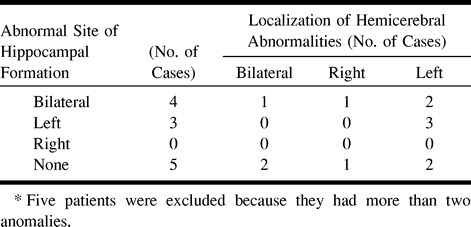
The relationship between the location of the hemicerebral abnormalities of heterotopia, polymicrogyria, and schizencephaly and the side (left, right, or bilateral) of the abnormal HF is shown in Table 2. Seventeen patients had either heterotopia, polymicrogyria, or schizencephaly; however, five patients were excluded because they had more than two anomalies, and the relationship was evaluated in the remaining 12 patients. When bilateral HFs were abnormal, localized cortical disorders were identified on either one or both hemispheres. In all three patients who had abnormal left HFs, the abnormal cerebral area was also located on the left side (Table 2).
TABLE 2:
Relationship between the laterality of abnormal hippo~campal formations and localized hemicerebral abnormalities in 12* patients with heterotopia, polymicrogyria, and schizencephaly
Discussion
Among the 44 patients with congenital malformations of the brain, abnormal HFs were observed in as many as 28 patients (64%) on coronal MR images. Agenesis of the corpus callosum is well known to be associated with varying degrees of limbic system malformations (10). The anterior commissure may be absent or hypoplastic, and fornices are usually laterally displaced or absent. Incomplete inversion of the HF is one such abnormality. Keyhole deformity of the temporal horns was also noted in association with a deficient HF (10). Similar hippocampal developmental malformations have also been detected in patients with lissencephaly and holoprosencephaly. Baker and Barkovich (6) examined the morphologic abnormalities of HF in patients with agenesis of the corpus callosum, lissencephaly, or holoprosencephaly and found abnormal vertical orientation of the HF in 82% of patients (eight of eight with agenesis of the corpus callosum, four of four with lissencephaly, and one of four with holoprosencephaly). Our study indicated an arrest of the normal process of hippocampal inversion in all patients with holoprosencephaly, agenesis of the corpus callosum, or lissencephaly. These hippocampal abnormalities were also noted in some patients with other diseases, such as heterotopia, polymicrogyria, schizencephaly, Dandy-Walker complex, tuberous sclerosis, and Fukuyama muscular dystrophy.
The biometric data are useful for evaluating selective hippocampal atrophy in patients with intractable partial seizures, Alzheimer-type dementia, amnesic syndromes, or schizophrenia. Obvious hippocampal atrophy on MR images corresponds to severe neuronal loss (11). To detect the abnormality, volumetric studies of the hippocampus have been performed. Normal hippocampal volumes have been found to be variable, ranging from 2.6 to 7.7 cm3 (11–15). Right hippocampal volumes are slightly greater than those on the left. Increases in total hippocampal volume were significantly correlated with age in children 15 to 83 months old (13) and in adolescents and young adults (15), owing to myelination and synaptogenesis.
The HF is the first cortical area to differentiate, and the hippocampal sulcus first appears in human fetuses at 10 weeks' gestation. As a result of the marked expansion of the neocortex and unequal growth of the various components of the hippocampus, there is gradual infolding of the components into a progressively smaller temporal horn. At 13 to 14 weeks, the unfolded hippocampus is located on the medial surface of the temporal lobe and the neocortex is located along the lateral aspect of the temporal lobe. The neocortex grows more rapidly than the allocortex, resulting in medial displacement and internal “inversion” of the dentate gyrus, hippocampus, and subiculum precursors into the temporal horn regions. At 15 to 16 weeks, the dentate gyrus and cornu ammonis start to infold. By 18 to 20 weeks, the hippocampus begins to resemble the adult hippocampus: the dentate gyrus and cornu ammonis have folded into the temporal lobe, the hippocampus and subiculum approximate each other across a narrow hippocampal sulcus, and the HF fissure becomes reoriented from vertical to horizontal (16).
Nearly all the diseases associated with hippocampal abnormalities in this study involved malformations of cerebral cortical development. Because hippocampal neurons have to migrate from a germinal zone to get to their proper final location, we presume that the abnormal hippocampal configurations in these patients are a result of abnormal hippocampal neuronal migration. Various hippocampal abnormalities were observed. Figure 1 (holoprosencephaly) shows vertical hippocampi that are normal in size. Figure 5 (tuberous sclerosis) shows small and vertically oriented HFs, which appear similar to those in a 16-week-old fetus (16). Figure 2 (Fukuyama muscular dystrophy) shows straight perpendicular HFs without hippocampal sulci, corresponding in appearance to those in a fetus 13 weeks old or younger. We also observed an abnormal globular-shaped HF (Fig 4), which we think resulted from an unfolded hippocampus or from arrested growth with or without the rudiment. Fukuyama muscular dystrophy is a severe form of such arrested growth.
In some studies of hippocampal developmental changes in patients with temporal lobe epilepsy (3, 17–19), abnormal HFs were observed in 1.7% to 7.2% of a global population of patients with partial epilepsy. These hippocampal abnormalities included incomplete rotation/translation, atrophy, round hippocampi, and an abnormally medial location, and were either isolated or associated with heterotopia, cerebral dysgenesis, or limited schizencephaly (3, 17–19). However, these studies were examined from the aspect of epilepsy. The present MR study indicates a high rate (64%) of vertical or globular HF in many kinds of congenital brain malformations.
HF changes are pronounced abnormalities in patients with congenital malformations, especially cortical disorders. These are sometimes difficult to detect, and one may miss subtle cortical disorders, such as polymicrogyria and heterotopia, on routine MR images. Therefore, it is important to use good T1-contrast MR images with thin slices. It has been reported that focal polymicrogyria cannot be identified by using an ordinary head coil but rather requires a special surface head coil (20). Our study demonstrated the laterality of abnormal sites of HF in focal cortical disorders. If coronal MR images show a unilateral abnormal HF, the focal cortical disorder would be located on the same side, causing epilepsy and/or developmental delay. HF is a visible component of a more extensive disorder, and the evaluation of HF on MR images may help in the detection of these abnormalities.
Conclusion
A high prevalence of abnormal HFs was observed in a wide variety of congenital malformations. Focusing on the morphologic abnormalities of HF on coronal MR images may help in the detection of brain anomalies, especially subtle cortical disorders.
Footnotes
Address reprint requests to Noriko Sato, MD, Department of Diagnostic Radiology, Gunma University School of Medicine, 3-39-15 Shouwa-machi, Maebashi, Gunma 371-8511 Japan.
References
- 1.Dowd CF, Dillon WP, Barbaro NM, Laxer KD. Intractable complex partial seizure: correlation of magnetic resonance imaging with pathology and electroencephalography. Epilepsy Res Suppl 1992;5:101-110 [PubMed] [Google Scholar]
- 2.Heinz ER, Crain BJ, Radtke RA, et al. MR imaging in patients with temporal lobe seizures: correlation of results with pathologic findings. AJNR Am J Neuroradiol 1990;11:827-832 [PMC free article] [PubMed] [Google Scholar]
- 3.Lehericy S, Dormont D, Semah F, et al. Developmental abnormalities of the medial temporal lobe in patients with temporal lobe epilepsy. AJNR Am J Neuroradiol 1995;16:617-626 [PMC free article] [PubMed] [Google Scholar]
- 4.Barkovich AJ, Koch TK, Carrol CL. The spectrum of lissencephaly: report of ten patients analyzed by magnetic resonance imaging. Ann Neurol 1991;30:139-146 [DOI] [PubMed] [Google Scholar]
- 5.Kier EL, Fulbright RK, Bronen RA. Limbic lobe embryology and anatomy: dissection and MR of the medial surface of the fetal cerebral hemisphere. AJNR Am J Neuroradiol 1995;16:1847-1853 [PMC free article] [PubMed] [Google Scholar]
- 6.Baker LL, Barkovich AJ. The large temporal horn: MR analysis in developmental brain anomalies versus hydrocephalus. AJNR Am J Neuroradiol 1992;13:115-122 [PMC free article] [PubMed] [Google Scholar]
- 7.Osborn AG, Boyer RS. Brain development and congenital malformations. In: Patterson AS, ed. Diagnostic Neuroradiology. St Louis: Mosby 1994:3-113 [Google Scholar]
- 8.Van-der-Knaap MS, Smit LM, Barth PG, et al. Magnetic resonance imaging in classification of congenital muscular dystrophies with brain abnormalities. Ann Neurol 1997;42:50-59 [DOI] [PubMed] [Google Scholar]
- 9.Barkovich AJ. Neuroimaging manifestations and classification of congenital muscular dystrophies. AJNR Am J Neuroradiol 1998;19:1389-1396 [PMC free article] [PubMed] [Google Scholar]
- 10.Atlas SW, Zimmerman RA, Bilaniuk LT, et al. Corpus callosum and limbic system: neuroanatomic MR evaluation of developmental anomalies. Radiology 1986;160:355-362 [DOI] [PubMed] [Google Scholar]
- 11.Hasboun D, Chantome M, Zouaoui A, et al. MR determination of hippocampal volume: comparison of three methods. AJNR Am J Neuroradiol 1996;17:1091-1098 [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobsen LK, Giedd JN, Castellanos FX, et al. Progressive reduction of temporal lobe structures in childhood-onset schizophrenia. Am J Psychiatry 1998;155:678-685 [DOI] [PubMed] [Google Scholar]
- 13.Szabo CA, Wyllie E, Siavalas EL, et al. Hippocampal volumetry in children 6 years or younger: assessment of children with and without complex febrile seizures. Epilepsy Res 1999;33:1-9 [DOI] [PubMed] [Google Scholar]
- 14.Aylward EH, Minshew NJ, Goldstein G, et al. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology 1999;53:2145-2150 [DOI] [PubMed] [Google Scholar]
- 15.Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry 1994;51:477-484 [DOI] [PubMed] [Google Scholar]
- 16.Kier EL, Kim JH, Fulbright RK, Bronen RA. Embryology of the human fetal hippocampus: MR imaging, anatomy, and histology. AJNR Am J Neuroradiol 1997;18:525-532 [PMC free article] [PubMed] [Google Scholar]
- 17.Baulac M, Grissac ND, Hasboun D, et al. Hippocampal developmental changes in patients with partial epilepsy: magnetic resonance imaging and clinical aspects. Ann Neurol 1998;44:223-233 [DOI] [PubMed] [Google Scholar]
- 18.Palmini A, Andermann F, Olivier A, et al. Focal neuronal migration disorders and intractable partial epilepsy: a study of 30 patients. Ann Neurol 1991;30:741-749 [DOI] [PubMed] [Google Scholar]
- 19.Kuzniecky R, Garcia JH, Faught E, Morawetz RB. Cortical dysplasia in temporal lobe epilepsy: magnetic resonance imaging correlations. Ann Neurol 1991;29:293-298 [DOI] [PubMed] [Google Scholar]
- 20.Grant PE, Barkovich AJ, Wald LL, Dillon WP, Laxer KD, Vigneron DB. High-resolution surface-coil MR of cortical lesions in medically refractory epilepsy: a prospective study. AJNR Am J Neuroradiol 1997;18:291-301 [PMC free article] [PubMed] [Google Scholar]