Abstract
BACKGROUND AND PURPOSE: The intravenous use of recombinant tissue-type plasminogen activator (rTPA) in acute ischemic stroke has been investigated in three large trials. Limited series have reflected outcome after local intraarterial thrombolysis (LIT) in the cerebral territory. The purpose of this study was to evaluate the safety and efficacy of combined intraarterial/intravenous thrombolysis using rTPA (actilyse) for acute ischemic stroke.
METHODS: Forty-five patients with acute onset of severe hemispheric stroke and without signs of major cerebral infarction on early CT scans were randomized by order of admission. Twelve patients were treated with 50 mg actilyse (maximal dose, 0.7 mg/kg); three had occlusion of the internal carotid artery and nine had occlusion of the middle cerebral artery. Thrombolysis was started by LIT and continued intravenously within 6 hours of stroke onset. Outcome, assessed after 1 and 12 months according to the modified Rankin scale (MRS), was considered good (MRS score, 0–3) for patients who were functionally independent and poor (MRS score, 4–5) for those who were dependent or had died.
RESULTS: In the thrombolysis group, outcome was good in eight patients at 1 month and in 10 patients at 12 months; in the control group, outcome was good in seven (21%) and 11 (33%) patients, respectively. Of the eight patients with a good outcome after thrombolysis, four had complete and one had partial recanalization. In the control group, the rate of intracerebral hemorrhage was 6%. Mortality at 1 month in the thrombolysis and control groups was 17% and 48%, respectively.
CONCLUSIONS: Combined intraarterial/intravenous thrombolysis with low-dose rTPA may be a safe and effective treatment for acute ischemic stroke within 6 hours in carefully selected patients.
One of the main objectives in the treatment of acute cerebral ischemia is the rapid restoration or improvement of blood flow in an affected vascular territory. Recombinant tissue-type plasminogen activator (rTPA) has been recognized as a promising agent because of its endogenous origin, short half-life in plasma, and high fibrin specificity, all of which promise to promote encouraging clinical results. However, the results of three large, randomized, placebo-controlled trials of thrombolytic therapy with intravenous rTPA have not been unequivocal (1–3). Some authors have reported a good outcome after local intraarterial thrombolysis (LIT) with different thrombolytic drugs (4–7) and others have described LIT followed by percutaneous transluminal angioplasty or carotid endarterectomy (8) or another intracranial manipulation (9) in limited series of stroke patients. The combined reported experience indicates that there are still at least three issues to be addressed before administration of rTPA becomes routine in patients with acute stroke: inclusion criteria, technical approach, and dose range. The purpose of this open-label study was to assess the safety and efficacy of combined intraarterial/intravenous administration of low-dose rTPA in patients with acute ischemic stroke.
Methods
During the period from February 1997 to March 1998, 612 patients with acute ischemic stroke were treated at our hospital, of whom 219 were admitted within 6 hours after onset of symptoms. All these patients underwent unenhanced CT studies, which were assessed by a neuroradiologist. If early CT scans showed such changes as sulcal effacement, mass effect, edema, or possible hemorrhage, the patient was excluded from the study, in accordance with reported guidelines (10).
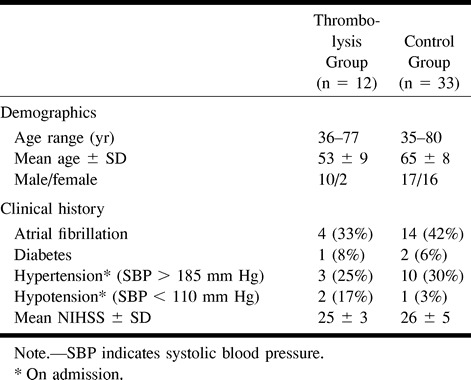
Corresponding to a study protocol approved by an institutional review committee, LIT was considered a possible treatment if it could be completed within 6 hours of stroke (5) and if the patient was not over 80 years old (11). To ensure a better match between treated patients and control subjects, only patients with symptoms referable to the internal carotid artery (ICA) territory were included. Exclusion criteria for thrombolysis were adopted from the National Institute of Neurological Disorders and Stroke (NINDS) study (1). Pretreatment examination revealed 45 eligible patients who presented with a severe but stable hemispheric syndrome who were then randomized to either the thrombolysis or control group, one after another, according to the order of admission. After informed consent requirements were completed, only 12 patients remained in the thrombolysis group whereas 33 patients were included in the conventional treatment, or control, group. Baseline characteristics of the patients in the two groups appear in Table 1. All patients had a score of 20 or higher on the National Institutes of Health Stroke Scale (NIHSS) (12) at the onset of treatment.
TABLE 1:
Baseline characteristics of the patients
The patients in the control group were transferred directly to the intensive care unit (ICU), while those in the thrombolysis group underwent cerebral angiography. Inclusion criteria for angiography required complete occlusion of the ICA or the middle cerebral artery (MCA) trunk (M1) or branch (M2) in the appropriate carotid territory, consistent with the patient's clinical presentation (13, 14). Among the 12 consecutive patients enrolled in the thrombolysis group, three had occlusion of the ICA and nine had occlusion of the MCA.
For the infusion, 50 mg of actilyse (Boehringer Ingelheim Pharma GmbH, Gaithersburg, MD) was dissolved in 50 mL of sterile water (dose rate, 0.57–0.70 mg/kg). Intraarterial injection of a 25-mg dose was performed manually over a period of 5 to 10 minutes and was located selectively depending on the site of arterial occlusion. If the MCA was thrombosed, a catheter was placed into the M1 origin. If the ICA was occluded extracranially, actilyse was injected into the arterial stump. There were no attempts to insert the catheter into the thrombus, and the catheter position was not changed during the injection. When the injection was completed, the patient was transferred to the ICU, where the remainder of the dose of actilyse (25 mg) was injected intravenously via an infusion pump over a period of 60 minutes. A femoral introducer was left in place until the following day, when CT and angiography were performed again.
All the patients included in the study received standard neurovascular care, including heparin at an initial dose of 5000 U and thereafter at 5000 U twice a day for several days, after which the patient was maintained on either aspirin or warfarin. If hemorrhagic conversion occurred, heparin was discontinued.
Evaluation of anatomic results and clinical outcome was based on findings on repeat CT studies, control angiograms, and neurologic examination performed during the first 24 hours, at the time of discharge from the hospital, at 1 month, and again at 12 months. Early neurologic improvement was classified as significant if the patient improved by four or more points on the NIHSS at 24 hours (11), although this was not recognized as the end point of the study. Arterial patency was rated by the thrombolysis in myocardial infarction classification scheme (15), in which 0 indicates no perfusion, 1 is penetration beyond obstruction but no perfusion of distal beds, 2 is incomplete recanalization with slower distal perfusion, and 3 is full perfusion.
Outcome was assessed according to the modified Rankin scale (MRS), which is a handicap scale (16) on which patients are graded with reference to their previous lifestyle from 0 (absence of symptoms) to 6 (death). Outcome was grouped into two categories: good (MRS score, 0–3) for those patients who were functionally independent, or poor for those who were dependent (MRS score, 4–5) or had died.
Results
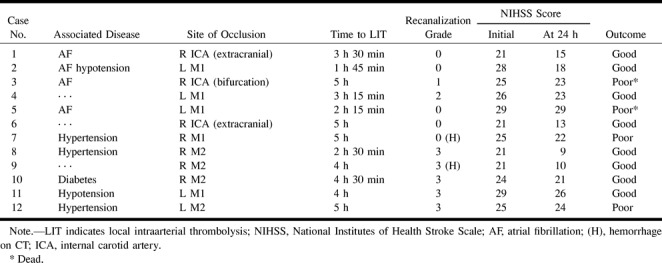
The protocol of thrombolysis was successfully completed in all patients without any side effects. Follow-up CT studies revealed hemorrhagic conversion in two patients (17%). In both cases, the hemorrhage was located in the core of the infarction with a diameter of less than 2 cm and was not associated with neurologic deterioration. Two other patients died within 4 days of progressive stroke and fatal swelling of the hemisphere, despite partial recanalization of an occluded artery in one of the patients. Comparison of baseline characteristics, anatomic results of thrombolysis, and outcome at 1 month (Table 2) showed that clinical improvement was not always associated with reopening of the occluded artery. However, complete recanalization was achieved in all M2 occlusions.
TABLE 2:
Comparison of baseline characteristics, anatomic results of thrombolysis, and outcome at 1 month
In the control group, hemorrhagic conversion occurred in two patients (6%) and one of them died. Within 1 month, 16 (48%) of the patients in the control group died (overall mortality for those admitted within 6 hours after stroke was 30%) and 10 remained functionally dependent. This made the outcome at 1 month poor for 26 (79%) of the patients in this group. An additional analysis of outcome by subgroups was performed among the control subjects for possible impact of age and location of stroke. The mean age in the good (n = 7), poor-disabled (n = 10), and poor-dead (n = 16) subgroups was 65 ± 9.7, 65 ± 5.9, and 65 ± 8.8 years, respectively. Therefore, the mean age across subgroups was regarded as similar. The rate of stroke in the left ICA territory for those who survived was 57% (four patients) in the good subgroup and 50% (five patients) in the poor-disabled subgroup.
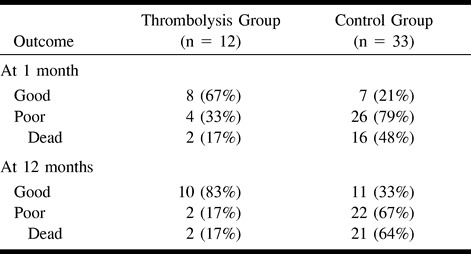
Comparison of outcomes in the study subgroups is presented in Table 3. Although the rate of hemorrhagic conversion in the thrombolysis group (17%) was much higher than that in the control group (6%), an adverse association between thrombolysis and poor outcome was observed. At 12 months, 83% of the patients in the thrombolysis group were functionally independent whereas only 33% of the control subjects had a good outcome.
TABLE 3:
Outcome in the study groups
Representative Cases
Case 4
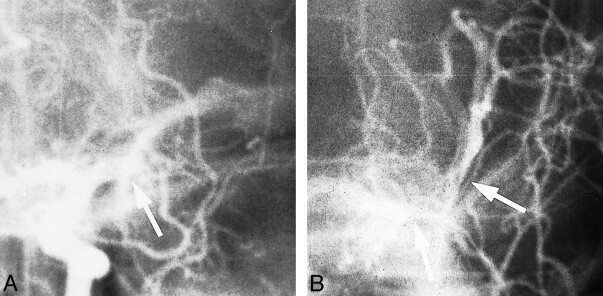
A 77-year-old man presented with sudden onset of right hemiplegia and global aphasia. He was not alert but arousable, and his baseline NIHSS score was 26. A CT scan on admission revealed no abnormalities. Angiography confirmed complete occlusion of the left MCA trunk (Fig 1A). LIT was initiated 3 hours 15 minutes after the onset of symptoms. Control angiography performed the next day revealed partial recanalization of the left M1 trunk with residual thrombus (Fig 1B) and slower distal perfusion. A follow-up CT study showed no cerebral edema; however, there was a low-density area in the territory of the left MCA. At 24 hours, positive neurologic changes occurred, with severe paresis in the arm and moderate paresis in the leg (NIHSS score, 23). After 1 month, there was moderate paresis in the arm, mild paresis in the leg, and moderate aphasia (MRS score, 3). Motor function and language skills were better (MRS score, 2) at the final follow-up at 12 months.
fig 1.
Case 4.
A and B, Frontal projection shows occlusion of left M1 trunk before thrombolysis (arrow, A) and partial recanalization of artery with remnant of thrombus on control angiogram (arrows, B).
Case 9
A 48-year-old man presented with a severe left hemiparesis, left homonymous hemianopia, and drowsiness. Results of an initial CT scan were normal. Angiography showed distal occlusion of the right MCA parietooccipital branch (Fig 2A). LIT was started 4 hours after the onset of stroke. A control angiogram revealed recanalization of the right M2 branch (Fig 2B), and a CT scan the next day showed a low-density area with a high-density core in the right MCA territory. At 24 hours, the patient's neurologic status had improved significantly, to minor left hemiparesis and partial hemianopia. An MR examination after 2 weeks showed three small infarctions in the territory of the former low-density area detected by CT: one (frontotemporal) with a trace of hemorrhage and two (parietal and parasagittal) without hemorrhage. The patient recovered well, and after 12 months there was no evidence of disability (MRS score, 0).
fig 2.
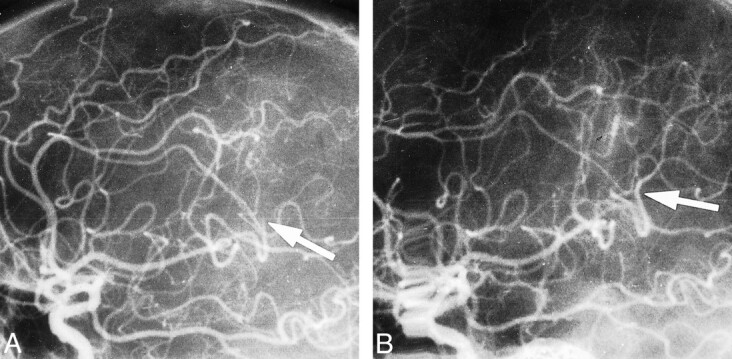
Case 9.
A and B, Lateral projection shows distal occlusion of M2 branch before thrombolysis (arrow, A) and complete recanalization on control angiogram (arrow, B).
Discussion
Since the first report on thrombolysis in the treatment of cerebral artery occlusion (17), early thrombolytic intervention in patients with acute ischemic stroke has been a continuing challenge. The most thoroughly studied thrombolytics are streptokinase and rTPA, followed by urokinase and pro-urokinase. Because three trials of streptokinase were stopped because of high rates of acute mortality and intracranial bleeding (18–20), clinical interest is presently focused on rTPA (21).
When administrated within 90 minutes of symptom onset, intravenous rTPA at doses of less than 0.95 mg/kg have proved relatively safe with regard to hemorrhagic complications (22). In the NINDS study (1), intravenous rTPA in a dose of 0.9 mg/kg (maximum, 90 mg) within 3 hours improved clinical outcome at 3 months despite an increased rate of symptomatic intracerebral hemorrhage, and in the European Cooperative Acute Stroke Study (ECASS II), treatment with the same dose within 6 hours of stroke onset was still as safe as within 3 hours, although no statistical benefit could be confirmed as compared with a placebo (3). The authors of the latter study concluded that the higher dose of rTPA may have contributed to better efficacy, and that individual characteristics, such as the site and extent of thrombus and collateral blood flow, may be relevant. However, ECASS I demonstrated that intravenous rTPA at higher doses leads to a higher mortality rate (2).
Intraarterial administration of rTPA helps to achieve a high concentration of the thrombolytic drug in the affected vascular territory without an excessive systemic dose rate. It has been reported that LIT with rTPA at doses of 20 to 40 mg is safe and effective within 6 hours of stroke onset and even beyond (4, 5, 8). In addition, angiography performed before LIT depicts vascular disorders and provides information on collateral flow.
In the present study, we decided to begin LIT treatment with the minimum dose required for therapeutic workup. The plasma half-life of rTPA is reported to be 5 minutes, but its biological activity, once bound to a clot, may last for several hours (23). Therefore, for the best efficacy, rTPA should be administrated as a continuous infusion after an initial bolus (24), which is why we combined LIT with a follow-up intravenous infusion of rTPA.
Although angiography itself very early in the course of cerebral ischemia is reported to be feasible and safe (13), the risk of complications increases with intraarterial selective catheterization and administration of a contrast agent (25). Such an increased risk may be acceptable on behalf of the state of the art, as in the one randomized, controlled, double-blind trial of LIT (26) and as needed to reach a high level of significance; however, the absence of a placebo was one reason why angiography was not included in our protocol for the control group.
Coadministration of rTPA and heparin appears to produce a contradictory effect. According to the recommendations of the American Heart Association (10), persons given intravenous rTPA in clinical practice should not receive antithrombotic or antiplatelet drugs within 24 hours of treatment (however, it was also stated that additional research on the usefulness of such adjunctive therapies is needed, because they may affect the time to lysis, degree of reperfusion, occurrence of reocclusion, and/or clinical outcome). In contrast, it has been shown that rTPA at a dose of 100 mg can be given intravenously together with heparin (5000 U bolus followed by 1000 U/h) with acceptable safety (9, 14). Indeed, subcutaneous heparin (not exceeding 10,000 U) was allowed during the first 24 hours in ECASS I and ECASS II for prophylaxis of deep-vein thrombosis (2, 3).
Intraarterial manipulation to reopen an occluded artery is usually performed just after intravenous administration of a 5000-U bolus of heparin (4, 27, 28); in the prolyse in acute cerebral thromboembolism trial, both groups received intravenous heparin (26). Therefore, in the present study, 5000 U of heparin after the first CT study, followed by 5000 U subcutaneously twice daily, produced a response to a modest coadministration of heparin for maintenance of intraarterial catheters and as another prophylaxis of thrombosis. Furthermore, recent findings in a clinical investigation suggest that heparin may play a role as an adjunctive treatment in the thrombolysis of stroke (29), as it does in myocardial infarction (30). Moreover, in another study, rTPA treatment for acute stroke was characterized by a massive and sustained increase in activation markers of coagulation (31), a finding that supports adjunctive anticoagulation for ischemic stroke treated with rTPA.
Our clinical research was intended to be a pilot study in which both with the thrombolysis and control groups received the best available therapy. However, our results failed to demonstrate a clear benefit for the therapeutic method because of the baseline differences between the groups (Table 1), which weakened the comparison and moved the study toward the format of a case series. On the other hand, the following discussion of the results may encourage further attempts to improve the efficacy of thrombolysis for patients with acute ischemic stroke.
The overall distribution of good outcome (MRS score, 0–3) at 1 month and at 12 months in the groups (thrombolysis/control) were 67%/21% and 83%/33%, respectively. A post hoc analysis of MRS scores in the ECASS II trial dichotomized for dependency (scores of 0, 1, and 2 were classified as favorable) revealed that 54% of patients in the rTPA group and 46% in the placebo group were independent at day 90. In the NINDS study, 61% of patients in the rTPA group and 51% in the placebo group had an MRS score of 0 to 3 at 3 months. Recent studies with LIT found an overall good outcome (defined as MRS scores of 0–3) at 3 months in 61% of patients (7) and at 6 months in 65% of patients (32). The good outcome at 1 month in our study is consistent with those reported before (1, 7). A notable absolute difference that might favor rTPA treatment may be the location of thrombus: in patients with MCA occlusions, outcome after LIT compared with a natural course showed a 30% increase in the number of patients with minimal or no disability (6).
Despite the relatively low dose of rTPA, hemorrhagic conversion was almost three times more common in the thrombolysis group than in the control group (17% versus 6%). However, this difference did not lead to increased mortality and there were no symptomatic intracerebral hemorrhages in the thrombolysis group. During the first 30 days, more deaths occurred in the control group relative to the thrombolysis group (48% versus 17%), and this difference was even more obvious at 12 months (64% versus 17%). After intravenous administration of rTPA within 6 hours of stroke onset, parenchymal hemorrhage was roughly four times more common in the rTPA group (12% versus 3%) in the ECASS II trial and similarly (9% versus 2%) within 3 hours in the NINDS study; there were no significant differences in mortality at 3 months between the rTPA and control groups in the ECASS II trial (10.5% versus 10.7%) or in the NINDS study (17% versus 21%) (1, 3). In a community-based approach (29), the rates of intracerebral hemorrhage and mortality after early (within 3 hours of onset) intravenous thrombolysis for acute ischemic stroke were reported to be 11% and 12%, respectively. After intravenous administration of rTPA within 1 hour 30 minutes (22) and within 8 hours (13) of stroke onset, the overall rates of hemorrhagic conversion/death at 30 days were 4%/8% and 31%/13%, respectively. Although the time to thrombolysis should be considered an important predictor of outcome and adverse events, a time-related association between hemorrhagic conversion and mortality after intravenous administration of rTPA within the reported hours and doses is not strong.
LIT in the carotid territory using rTPA with a mean time delay from 4 hours 40 minutes to 6 hours 30 minutes (in different subgroups) was reported to be associated with 18 cases (13%) of hemorrhage (one symptomatic) and seven deaths (5%) in a series of 142 patients (5); in no patient did secondary hemorrhagic transformation (six cases, 18%) cause clinical deterioration, and eight patients (24%) died after LIT for a carotid territory stroke within 6 hours of onset (4). However, if an occlusion of the ICA bifurcation was treated with intraarterial rTPA, reported mortality ranged from 53% to 62% (8, 9).
The rates of adverse events and mortality observed after combined intraarterial/intravenous thrombolysis may be estimated according to reported results of intravenous thrombolysis and LIT. The rates of hemorrhagic conversion in both study groups may be acceptable and consistent with the experience discussed above (1, 3, 4, 29). Conversely, the rates of poor outcome and mortality in the control group were considerably higher relative to those in the control groups in related studies. Several reasons could account for this difference. First, a remarkable difference between mean ages in the groups was suspected. Nevertheless, the post hoc analysis of age in the control group revealed that mean ages in the outcome subgroups (good, poor-disabled, and poor-dead) were similar. Second, the percentage of left-sided strokes in which cognition was impaired was tested because of the possible impact on quality of outcome in survivors. Again, there were more strokes in the left ICA territory in the good subgroup than in the poor-disabled subgroup (57% versus 50%). Clinical history may be another factor in the different outcomes in the groups, as suggested by the natural course of patients with a stable and severe ischemic hemispheric syndrome of sudden onset. Usually caused by carotid T occlusion, M1 occlusion, or distal MCA occlusion, this syndrome is associated with high rates of major morbidity, with mortality rates as high as 55% in some series (6, 8, 9), and in patients with massive strokes, mortality may be as high as 70% to 100% at 3 months (33).
The primary goal of thrombolysis in stroke patients should be the rapid restoration of blood supply. However, data evaluating the efficacy of thrombolysis based only on the revascularization rate of occluded arteries are not sufficiently accurate, because an artery can be reopened yet not efficient (4, 5, 8). Conversely, a good clinical outcome may be obtained even when an artery remains occluded, because of thrombolysed collaterals (5, 34, 35). Our study confirmed the rapid reopening of an occluded artery as a predictor of outcome in 67% of patients: in five patients recanalization (one partial) resulted in a good outcome and in three patients nonrecanalization resulted in a poor outcome. Results in the remaining 33% of patients supported the opinion that findings on a control angiogram after thrombolysis could be elusive in regard to outcome. Another pilot study showed that combined intraarterial/intravenous thrombolysis is feasible and provides better recanalization, although it was not associated with improved clinical outcome (36).
The foregoing may allow us to expect that the workup for intraarterial administration of rTPA, recognized at present as a limitation of LIT, could be reduced to a minimum, consisting of an intraarterial injection under fluoroscopic guidance. However, patients with a T-type occlusion of the ICA may experience little potential benefit from thrombolysis (4, 7, 9, 37, 38) and may be excluded on the basis of findings at sonography, CT angiography, or MR angiography (22, 38–40).
Conclusion
The present study indicates that combined intraarterial/intravenous thrombolysis with low-dose rTPA may be a safe and effective treatment for acute ischemic stroke if administered within 6 hours of onset in carefully selected patients. However, because of the differences in baseline characteristics between our groups and the small number of cases, further research is needed to assess the significance of this observation.
Footnotes
Supported in part by the Medical Academy of Latvia grant 96.0346.
Address reprint requests to Valdis Keris, MD, Department of Neurosurgery, Medical Academy of Latvia, Clinical Hospital “Gailezers,” 2, Hipokrata St, LV-1038 Riga, Latvia.
References
- 1. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581-1587 [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017-1025 [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet 1998;352:1245-1251 [DOI] [PubMed] [Google Scholar]
- 4.Zeumer H, Freitag H-J, Zanella F, Thie A, Arning C. Local intra-arterial fibrinolytic therapy in patients with stroke: urokinase versus recombinant tissue plasminogen activator (r-TPA). Neuroradiology 1993;35:159-162 [DOI] [PubMed] [Google Scholar]
- 5.Theron J, Coskun O, Huet H, Oliveira G, Toulas P, Payelle G. Local intraarterial thrombolysis in the carotid territory. Intervent Neuroradiol 1996;2:111-126 [DOI] [PubMed] [Google Scholar]
- 6.Bendszus M, Urbach H, Ries F, Solymosi L. Outcome after local intra-arterial fibrinolysis compared with the natural course of patients with a dense middle cerebral artery on early CT. Neuroradiology 1998;40:54-58 [DOI] [PubMed] [Google Scholar]
- 7.Gonner F, Remonda L, Mattle H, et al. Local intra-arterial thrombolysis in acute ischemic stroke. Stroke 1998;29:1894-1900 [DOI] [PubMed] [Google Scholar]
- 8.Endo S, Kuwayama N, Hirashima Y, Akai T, Nishijima M, Takaku A. Results of urgent thrombolysis in patents with major stroke and atherothrombotic occlusion of the cervical internal carotid artery. AJNR Am J Neuroradiol 1998;19:1169-1175 [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen O, von Kummer R, Forsting M, Hacke W, Sartor K. Thrombolytic therapy in acute occlusion of the intracranial internal carotid artery bifurcation. AJNR Am J Neuroradiol 1995;16:1977-1986 [PMC free article] [PubMed] [Google Scholar]
- 10. American Heart Association. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Circulation 1996;4:1167-1174 [DOI] [PubMed] [Google Scholar]
- 11.Haley EC Jr, Brott TG, Sheppard GL, et al. Pilot randomized trial of tissue plasminogen activator in acute ischemic stroke. Stroke 1993;24:1000-1004 [DOI] [PubMed] [Google Scholar]
- 12.Brott TG, Adams HP, Olinger CP, et al. Measurement of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864-870 [DOI] [PubMed] [Google Scholar]
- 13.del Zoppo GJ, Poeck K, Pessin MS, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992;32:78-86 [DOI] [PubMed] [Google Scholar]
- 14.von Kummer R, Hacke W. Safety and efficacy of intravenous tissue plasminogen activator and heparin in acute middle cerebral artery stroke. Stroke 1992;22:646-652 [DOI] [PubMed] [Google Scholar]
- 15.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in myocardial infarction (TIMI) trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase: clinical findings through hospital discharge. Circulation 1987;76:142-154 [DOI] [PubMed] [Google Scholar]
- 16.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJA, van Gijn J. Inter-observer agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-607 [DOI] [PubMed] [Google Scholar]
- 17.Sussmann BJ, Fitch TSP. Thrombolysis with fibrinolysis in cerebral artery occlusion. JAMA 1958;167:1705-1709 [DOI] [PubMed] [Google Scholar]
- 18.Donnan GA, Davis SM, Chambers BR, et al. Trials of streptokinase in severe acute ischemic stroke. Lancet 1995;345:578-579 [PubMed] [Google Scholar]
- 19. Multicentre Acute Stroke Trial-Italy (MAST-I) Group. Randomised controlled trial of streptokinase, aspirin, and combination of both in treatment of acute ischemic stroke. Lancet 1995;346:1509-1514 [PubMed] [Google Scholar]
- 20. Multicenter Acute Stroke Trial-Europe Study Group. Thrombolytic therapy with streptokinase in acute ischemic stroke. N Engl J Med 1996;335:145-150 [DOI] [PubMed] [Google Scholar]
- 21.Fisher M, Bogousslavsky J. Further evolution toward effective therapy for acute ischemic stroke. JAMA 1998;279:1298-1303 [DOI] [PubMed] [Google Scholar]
- 22.Brott TG, Haley EC Jr, Levy DE, et al. Urgent therapy for stroke, part I: pilot study of tissue plasminogen activator administrated within 90 minutes. Stroke 1992;23:632-640 [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg PR, Sherman LA, Tiefenbrunn AJ, Ludbrook PA, Sobel BE, Jaffe AS. Sustained fibrinolysis after administration of t-PA despite its short half-life in the circulation. Thromb Haemost 1987;57:35-40 [PubMed] [Google Scholar]
- 24.Silver B, Weber J, Fisher M. Medical therapy for ischemic stroke: review. Clin Neuropharmacol 1996;19:101-128 [DOI] [PubMed] [Google Scholar]
- 25.Culebras A, Kase CS, Masedu JC, et al. Practice guidelines for the use of imaging in transient ischemic attacks and acute stroke: a report of Stroke Council, American Heart Association. Stroke 1997;28:1480-1497 [DOI] [PubMed] [Google Scholar]
- 26.del Zoppo GJ, Higashida RT, Furlan AJ, et al. The prolyse in acute cerebral thromboembolism trial (PROACT): results of 6 mg dose tier. Stroke 1996;27:164 [Google Scholar]
- 27.Nakano S, Yokogami K, Ohta H, Yano T, Ohnishi T. Direct percutaneous transluminal angioplasty for acute middle cerebral artery occlusion. AJNR Am J Neuroradiol 1998;19:767-772 [PMC free article] [PubMed] [Google Scholar]
- 28.Levy DI. Endovascular treatment of carotid artery occlusion in progressive stroke syndromes: technical note. Neurosurgery 1998;42:186-193 [DOI] [PubMed] [Google Scholar]
- 29.Grond M, Stenzel C, Schmulling S, et al. Early intravenous thrombolysis for acute ischemic stroke in a community-based approach. Stroke 1998;29:1544-1549 [DOI] [PubMed] [Google Scholar]
- 30.Gunnar RM, Passamani ER, Bourdillon PDV, et al. Guidelines for the early management of patients with acute myocardial infarction: a report of the American College of Cardiology/American Heart Association task force on assessment of diagnostic and therapeutic cardiovascular procedures. J Am Coll Cardiol 1990;16:249-292 [DOI] [PubMed] [Google Scholar]
- 31.Fassbender K, Dempfle CE, Mielke O, et al. Changes in coagulation and fibrinolysis markers in acute ischemic stroke treated with recombinant tissue plasminogen activator. Stroke 1999;30:2101-2104 [PubMed] [Google Scholar]
- 32.Ueda T, Sakaki S, Kumon Y, Ohta S. Multivariable analysis of predictive factors related to outcome at 6 months after intra-arterial thrombolysis for acute ischemic stroke. Stroke 1999;30:2360-2365 [DOI] [PubMed] [Google Scholar]
- 33.Clark WM, Albers GW, Madden KP, Hamilton S, for the Thrombolytic Therapy in Acute Ischemic Stroke Study Investigators. The rtPA (Alteplase) 0- to 6-hour acute stroke trial, part A (A0276g). Stroke 2000;31:811-816 [DOI] [PubMed] [Google Scholar]
- 34.Herderschee D, Limburg M, van Royen EA, Hijdra A, Buller HR, Koster PA. Thrombolysis with recombinant tissue plasminogen activator in acute ischemic stroke: evaluation with rCBF-SPECT. Acta Neurol Scand 1991;83:317-322 [DOI] [PubMed] [Google Scholar]
- 35.Grotta JC, Alexandrov AV. tPA-associated reperfusion after acute stroke demonstrated by SPECT. Stroke 1998;29:429-432 [DOI] [PubMed] [Google Scholar]
- 36.Lewandowski CA, Frankel M, Tomsick TA, et al. Combined intravenous and intra-arterial r-TPA versus intra-arterial therapy of acute ischemic stroke. Stroke 1999;30:2598-2605 [DOI] [PubMed] [Google Scholar]
- 37.Kucinski T, Koch C, Grzyska U, Freitag H-J, Kromer H, Zeumer H. The predictive value of early CT and angiography for fatal hemispheric swelling in acute stroke. AJNR Am J Neuroradiol 1998;19:839-846 [PMC free article] [PubMed] [Google Scholar]
- 38.Wildermuth S, Knauth M, Brandt T, Winter R, Sartor K, Hacke W. Role of CT angiography in patient selection for thrombolytic therapy in acute hemispheric stroke. Stroke 1998;29:935-938 [DOI] [PubMed] [Google Scholar]
- 39.Kaps M, Link A. Transcranial sonographic monitoring during thrombolytic therapy. AJNR Am J Neuroradiol 1998;19:758-760 [PMC free article] [PubMed] [Google Scholar]
- 40.Gillard JH, Oliverio PJ, Barker PB, Oppenheimer SM, Bryan RN. MR angiography in acute cerebral ischemia of the anterior circulation: a preliminary report. AJNR Am J Neuroradiol 1997;18:343-350 [PMC free article] [PubMed] [Google Scholar]