Abstract
BACKGROUND AND PURPOSE: The natural history of asymptomatic major cerebral artery occlusive disease is unclear. Rate of symptomatic change, appearance of new lesions on MR images, and cerebral hemodynamics were analyzed for patients with asymptomatic major cerebral artery occlusion.
METHODS: This prospective study included asymptomatic patients who had occlusive disease between 1992 and 1995. MR imaging and MR angiography were used to detect internal carotid artery (ICA) or middle cerebral artery (MCA) occlusion in 3965 neurologically asymptomatic patients and for follow-up of affected patients for 67 to 105 months (mean, 79 months). Regional cerebral blood flow and cerebrovascular reserve capacity were examined by xenon-enhanced CT at rest and after the administration of acetazolamide, respectively.
RESULTS: Eighteen patients had MCA occlusion and 17 had ICA occlusion. During the follow-up period, five patients became symptomatic (four with MCA occlusion and one with ICA occlusion), with no significant difference (P = .332) in the rate of symptomatic change. Among these five patients, new infarction occurred on the ipsilateral side in three patients, contralateral side in one, and bilateral sides in one. New stenotic or occlusive changes occurred in three patients. The patients were divided into groups: group A, without new lesions on MR images (n = 23), and group B, with new lesions (n = 12). There was no significant difference in regional cerebral blood flow value between groups A and B in the whole hemisphere, anterior cerebral artery territory, or MCA territory. There was a significant difference in cerebrovascular reserve capacity between groups A and B between the affected side (P = .00051 and P = .00068, respectively) and the contralateral side (P = .00101 and P = .00115, respectively) for the whole hemisphere and MCA territory, and the difference was more severe on the affected side in both regions.
CONCLUSION: These pilot data suggest that asymptomatic MCA occlusion has a worse prognosis than does ICA occlusion. Silent events are common bilaterally. This may be because of hemodynamic factors or perhaps MCA occlusion is a marker for a more progressive type of atherosclerosis. A prospective study involving assessment of hemodynamics and baseline stroke risk factors in patients with MCA occlusion is indicated.
The use of MR angiography and medical screening for brain diseases has increased the detection of asymptomatic major cerebral artery (internal carotid artery [ICA] and middle cerebral artery [MCA]) diseases in Japan (1–6). However, the indications for the treatment of such patients are difficult to determine. Establishment of the best preventative therapy requires that the natural history of the disease first be elucidated. The natural history of asymptomatic occlusive diseases detected by different imaging techniques has been described (7–13). Some patients, even with asymptomatic occlusion, show increased oxygen extraction fraction on positron emission CT scans (14), and such patients may be a subgroup of patients who are at high risk for subsequent stroke (15). This study examined the rate of symptomatic change, appearance of new lesions by MR imaging or MR angiography, and regional cerebral blood flow (rCBF) and cerebrovascular reserve capacity (CVR) by xenon-enhanced CT (Xe-CT) in patients with asymptomatic ICA and MCA occlusive disease.
Methods
With this prospective study, we examined asymptomatic patients with major cerebral artery occlusion. MR angiography and MR imaging were used to examine 3965 patients with no neurologic deficit, who were admitted for either screening for brain diseases or follow-up from 1992 to 1995. All patients with asymptomatic (no neurologic deficit but dizziness and headache are included) major cerebral artery occlusion were included in this study and were followed up until September 1999, for a period of 67 to 105 months (mean, 79 months). Patients without occlusion revealed by MR angiography were excluded from this study. None of these patients had a history of cerebrovascular disease or cardiovascular disorder. Patients with cerebral infarction or grade III or IV white matter lesions (16) and leukoaraiosis were excluded from this study. Informed consent for MR imaging, MR angiography, Xe-CT, and digital subtraction angiography was obtained from all patients, and the investigation was conducted in accordance with the guidelines of the Declaration of Helsinki.
The MR imaging method was described previously (16). Briefly, MR imaging, performed with a 1.5-T superconductive MR system, involved a spin-echo protocol for T1-weighted (600/15 [TR/TE]) and T2-weighted (3000/80) imaging in the orbitomeatal plane with 7.5-mm section thickness. The display matrix was 256 × 256. MR angiograms were obtained with the 3D time-of-flight method.
Xe-CT used a mixture of 30% xenon in oxygen inhaled for 3 min. During inhalation, a series of 8-s CT scans was obtained parallel to the orbitomeatal line. Regions of interest (ROI) were selected in each hemisphere, the anterior cerebral artery territory, and the MCA territory. An image-processing system was used to calculate the rCBF with the end-tidal chamber scan method. The confidential image was examined to detect motion artifacts, and a 6 × 6 filter was used for smoothing (17). Xe-CT was performed after the administration of 1000 mg of acetazolamide to measure the CVR. The CVR was calculated from the rCBF under acetazolamide stress minus the rCBF at rest. Digital subtraction angiography was performed to confirm the occlusion shown on MR angiograms.
Statistical comparison of the rate of symptomatic change between patients with MCA occlusion and those with ICA occlusion was conducted by using the Fisher exact test. Statistical analysis of rCBF and CVR data was conducted by using Student's t test. P < .05 was considered statistically significant.
Results
Asymptomatic major cerebral artery occlusion was detected in a total of 35 patients. Eighteen patients (11 men and seven women; mean age, 61.5 years) had MCA occlusion, and 17 patients (14 men and three women; mean age, 63.4 years) had ICA occlusion. The occlusion was on the left in 12 patients, on the right in 22 patients, and bilateral in one patient. MR examination was performed to screen for brain diseases in 15 patients, dizziness in nine, chronic headache in six, and hypertension in five. Twenty patients had histories of hypertension and six of diabetes mellitus, and 24 patients had received medication unrelated to antiplatelet therapy. MR imaging was performed two to seven times (mean, 3.8 times), and MR angiography was performed two to eight times (mean, 3.5 times) for each patient. Digital subtraction angiography was performed in 22 patients, and the findings were in concordance with those of MR angiography (10 patients with ICA occlusion and 12 patients with MCA occlusion).
During the follow-up period, five patients became symptomatic (Table 1). Four patients had MCA occlusion, and one had ICA occlusion. There was no significant difference in the rate of symptomatic change between the patients with MCA occlusion and those with ICA occlusion (P = .332, Fisher's exact test). The interval from diagnosis to symptomatic change varied from 24 to 52 months (mean, 32.4 months).
TABLE 1:
Patients with symptomatic change
MR imaging of the five patients who became symptomatic showed that the new infarction occurred on the ipsilateral side in three patients, the contralateral side in one, and bilaterally in one. MR angiography showed that new stenotic or occlusive changes occurred in three patients. Xe-CT revealed that three patients had poor response to acetazolamide and two had bilateral reduction of rCBF. Another seven patients who remained asymptomatic (five with MCA occlusion and two with ICA occlusion) were shown to have new infarction. Nine (50%) of the 18 patients with MCA occlusion had hemodynamic compromise, but only three (17.6%) of the 17 patients with ICA occlusion had hemodynamic compromise. Although the rate of hemodynamic compromise with MCA occlusion seems to be high, no significant difference was recognized (P = .075).
Based on analysis of cerebral hemodynamics, the patients were divided into two subgroups: patients without new lesion on MR images (group A, n = 23) and patients with new lesions (group B, n =12), including five patients with symptomatic change. There was no significant difference in mean hemispherical rCBF value in the affected and contralateral sides between groups A and B at any ROI. On the other hand, there was a significant difference in CVR between groups A and B on both the affected side (P = .00051 and P = .00068, respectively) and the contralateral side (P = .00101 and P = .00115, respectively) in both the hemispherical and MCA territory ROI. Moreover, the difference was greater on the affected side (Table 2). Typical findings are shown in Figures 1 and 2.
TABLE 2:
Values of rCBF and CVR in two groups
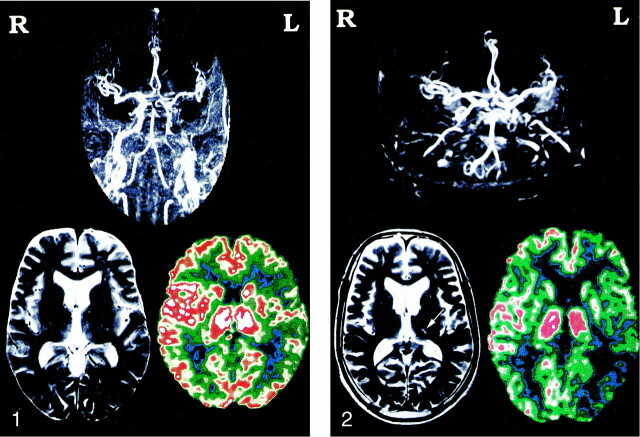
fig 1.
Representative case of a 55-year-old man. MR angiogram shows left ICA artery occlusion, but MR image is normal. Xe-CT image with acetazolamide shows poor response in the left hemisphere.fig 2. The same patient presented with hemisensory disturbance 2 years later. MR angiogram shows a new occlusion of the right ICA, and MR image shows a small infarction in the left thalamus (arrow). Xe-CT scan with acetazolamide shows poor response on both sides
Discussion
Previous studies of asymptomatic ICA occlusion used Doppler sonography (7–9), angiography (10–12), and either Doppler sonography, MR angiography, or angiography (13) for the diagnosis. Follow-up duration ranged from 27.4 to 52 months The incidence of symptomatic change varied from 0% to 16.7%. Three reports described rCBF evaluation (11–13), but there was no description of changes in MR imaging appearance. Long-term follow-up of patients with asymptomatic MCA occlusion has not been achieved. Recently, benign short-term outcome after asymptomatic carotid artery occlusion was reported (18), with ischemic stroke occurring in only one (3.3%) of 30 asymptomatic patients during an average follow-up duration of 32 months.
In our study, the follow-up period of 79 months with MR angiography and angiography detected only one patient with ICA occlusion that became symptomatic (5.9%). This rate is not much different from those of previous studies with short-term follow-up (7–13). However, the rate of symptomatic change for patients with MCA occlusion (22%) was higher than that for patients with ICA occlusion. Although the reason for the higher rate of symptomatic change in patients with MCA occlusion is unclear, we speculate that there are three possible channels of collateral flow in patients with ICA occlusion (through the anterior communicating artery, through the posterior communicating artery, and by leptomeningeal flow), whereas there is only one channel of collateral flow from leptomeningeal anastomosis in patients with MCA occlusion. The interval from diagnosis to symptomatic change was unclear in previous studies. We found that the interval was 24 to 52 months (mean, 32 months) and conclude that follow-up examination is necessary for at least 3 years after the diagnosis of occlusion is established.
MR imaging detected the responsible lesion in all five patients with symptomatic change. MR angiography showed the new stenotic lesion in two patients and the new occlusion in one.
The lower risk of stroke in asymptomatic patients was associated with a lower incidence of high oxygen extraction fraction (four of 30 patients) in contrast to symptomatic patients (39 of 81 patients) (P = .002). This finding further supports the importance of hemodynamic factors in the pathogenesis of ischemic stroke in patients with carotid artery occlusion (18). Comparison of rCBF and CVR in patients with asymptomatic (n = 14) and symptomatic ICA occlusion (n = 18) by use of Xe-CT showed that rCBF was not significantly different between the two groups but that CVR was significantly different, indicating that hemodynamic conditions are stable in patients with asymptomatic ICA occlusion (12). In contrast, examination of nine patients with asymptomatic occlusion (six cases of ICA occlusion and three cases of MCA occlusion) by single photon emission CT disclosed that four of the nine patients had cerebral hypoperfusion and seven of the nine had impaired CVR, so that patients with asymptomatic occlusion should be followed up as carefully as symptomatic patients (11). Our study showed that patients with new lesions revealed by MR imaging had significantly more impaired CVR than did patients with normal results of their MR imaging, and the degree of impairment was higher ipsilateral to the occlusion. Five previous studies have shown that patients with compromised CBF have a worse prognosis than do similar patients with normal CBF (19–23). Recently, impairment of CVR to hypercapnia was found to be significantly associated with an increased risk of ischemic events ipsilateral to carotid artery occlusion (23). Also, a significant correlation between ipsilateral ischemic events and diminished or exhausted CO2 reactivity has been shown (19). However, all five studies used different methods of hemodynamic assessment, which poorly correlate (24), so at present, it is safe to say that the physiological and clinical significance of an impaired blood flow response to acetazolamide is unknown.
In conclusion, patients with asymptomatic occlusion of the ICA have a better prognosis than do patients with asymptomatic occlusion of the MCA. Because five of 12 patients with new lesions revealed by MR imaging became symptomatic, intensive follow-up is necessary if CBF study indicates severe impairment of the CVR.
Footnotes
Address reprint requests to Nobuhiko Miyazawa, MD, Department of Neurosurgery, Yamanashi Medical University, 1110 Shimokatoh, Tamaho-machi, Nakakoma-gun, Yamanashi, 409-3898, Japan.
References
- 1.Kobayashi S, Okada K, Yamashita K. Incidence of silent lacunar lesion in normal adults and its relation to cerebral blood flow and risk factors. Stroke 1991;22:1379-1383 [DOI] [PubMed] [Google Scholar]
- 2.Matsubayashi K, Shimada K, Kawamoto A, Ozawa T. Incidental brain lesions on magnetic resonance imaging and neurobehavioral functions in the apparently healthy elderly. Stroke 1992;23:175-180 [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa T, Hashi K. The incidence and treatment of asymptomatic, unruptured cerebral aneurysms. J Neurosurg 1994;80:217-223 [DOI] [PubMed] [Google Scholar]
- 4.Hougaku H, Matsumoto M, Handa N, et al. Asymptomatic carotid lesions and silent cerebral infarction. Stroke 1994;25:566-570 [DOI] [PubMed] [Google Scholar]
- 5.Shinkawa A, Ueda K, Kiyohara Y, et al. Silent cerebral infarction in a community-based autopsy series in Japan: The Hisayama Study. Stroke 1995;26:380-385 [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S, Okada K, Koide H, Bokura H, Yamaguchi S. Subcortical silent brain infarction as a risk factor for clinical stroke. Stroke 1997;28:1932-1939 [DOI] [PubMed] [Google Scholar]
- 7.Hennerici M, Hülsbömer HB, Rautenberg W, Hefter H. Spontaneous history of asymptomatic internal carotid occlusion. Stroke 1986;17:718-722 [DOI] [PubMed] [Google Scholar]
- 8.Bornstein NM, Norris JW. Benign outcome of carotid occlusion. Neurology 1989;39:6-8 [DOI] [PubMed] [Google Scholar]
- 9.Bock RW, Gray-Weale AC, Mock PA, Robinson DA, Lusby RJ. The natural history of asymptomatic carotid artery disease. J Vasc Surg 1993;17:160-171 [DOI] [PubMed] [Google Scholar]
- 10.Nonino F, D'Alessandro R, Pazzaglia P, Conti E, Amato A, Borgatti E. Long term prognosis of asymptomatic carotid artery occlusion. Acta Neurol (Napoli) 1993;15:189-193 [PubMed] [Google Scholar]
- 11.Nakagawara J. Cerebral hemodynamics [in Japanese]. Gendai Iryou 1994;26:69-74 [Google Scholar]
- 12.Kashiwagi S, Yoshikawa K, Kawakami K, et al. Cerebral hemodynamics in patients with unilateral internal carotid artery occlusion [in Japanese, English abstr]. Jpn J Cereb Blood Flow Metab 1998;10:9-13 [Google Scholar]
- 13.Derdeyn CP, Yundt KD, Videen TO, Carpenter DA, Grubb RL, Powers WJ. Increased oxygen extraction fraction is associated with prior ischemic events in patients with carotid occlusion. Stroke 1998;29:754-758 [DOI] [PubMed] [Google Scholar]
- 14.Derdeyn CP, Vidden TO, Fritsch SM, Carpenter DA, Grubb RL, Powers WJ. Compensatory mechanisms for chronic cerebral hypoperfusion in patients with carotid occlusion. Stroke 1999;30:1019-1024 [DOI] [PubMed] [Google Scholar]
- 15.Grubb RL, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998;280:1055-1060 [DOI] [PubMed] [Google Scholar]
- 16.Miyazawa N, Satoh T, Hashizume K, Fukamachi A. Xenon contrast CT-CBF measurements in high-intensity foci on T2-weighted MR images in centrum semiovale of asymptomatic individuals. Stroke 1997;28:984-987 [DOI] [PubMed] [Google Scholar]
- 17.Miyazawa N, Mitsuka S, Asahara T, et al. Clinical features of relative focal hyperperfusion in patients with intracerebral hemorrhage detected by contrast-enhanced xenon CT. AJNR Am J Neuroradiol 1998;19:1741-1746 [PMC free article] [PubMed] [Google Scholar]
- 18.Powers WJ, Derdeyn CP, Fritsch SM, et al. Benign prognosis of never-symptomatic carotid occlusion. Neurology 2000;54:878-882 [DOI] [PubMed] [Google Scholar]
- 19.Kleiser B, Widder B. Course of carotid artery occlusion with impaired cerebrovascular reactivity. Stroke 1992;23:171-174 [DOI] [PubMed] [Google Scholar]
- 20.Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg 1993;79:483-489 [DOI] [PubMed] [Google Scholar]
- 21.Webster MW, Makaroun MS, Steed DL, Smith HA, Johnson DW, Yonas H. Compromised cerebral blood flow reactivity is a predictor of stroke in patients with symptomatic carotid artery occlusive disease. J Vasc Surg 1995;21:338-345 [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi H, Fukuyama H, Nagahama Y, et al. Evidence for misery perfusion and risk for recurrent stroke in major cerebral artery occlusive diseases from PET. J Neurol Neurosurg Psychiatry 1996;61:18-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vernieri F, Pasqualetti P, Passarelli F, Rossini PM, Silvestrini M. Outcome of carotid artery occlusion is predicted by cerebrovascular reactivity. Stroke 1999;30:593-598 [DOI] [PubMed] [Google Scholar]
- 24.Derdeyn CP, Grubb RL, Powers WJ. Cerebral hemodynamic impairment: methods of measurement and association with stroke risk. Neurology 1999;53:251-259 [DOI] [PubMed] [Google Scholar]