Abstract
BACKGROUND AND PURPOSE: The use of MR angiography and contrast-enhanced T1-weighted MR imaging in cases of acute cerebral ischemia may be helpful in the evaluation of middle cerebral artery (MCA) occlusion and leptomeningeal collaterals, respectively. The aim of our work was to investigate the relationship between MCA occlusion, T1-weighted vascular contrast enhancement, hemodynamic alterations, and tissue damage in cases of acute ischemic stroke.
METHODS: We studied the MCA territory in 15 patients with acute ischemic stroke within 8 hr of symptom onset. The first MR imaging study (<8 hr after onset) comprised diffusion-weighted imaging, MR angiography, perfusion-weighted imaging, and contrast-enhanced T1-weighted MR imaging sequences. Follow-up MR imaging, performed 1 week later, consisted of MR angiography and T2-weighted fluid-attenuated inversion recovery MR imaging.
RESULTS: Early MR angiography showed MCA stem occlusion in nine of 15 patients. Patients with MCA occlusion had significantly larger areas of abnormality on early diffusion-weighted images, significantly larger areas of altered hemodynamics, larger final lesion volumes, and poorer clinical outcome. Among the nine patients with MCA stem occlusion, vascular enhancement was marked in seven and absent in two who had complete MCA infarcts and poor clinical outcome. Among patients with MCA patency, vascular enhancement was marked in only one, mild in four, and absent in one. Patients with marked vascular enhancement had significantly larger regions of altered hemodynamics and significantly higher asymmetries in both regional cerebral blood volume and mean transit time because of increased values in the affected hemisphere.
CONCLUSION: Among patients with stroke with MCA occlusion, marked vascular enhancement and increased blood volume indicate efficient leptomeningeal collaterals and compensatory hemodynamic mechanisms.
The advent of new MR imaging sequences, such as diffusion- and perfusion-weighted imaging, has opened a new era in the early diagnosis of acute stroke. Diffusion-weighted imaging allows identification of the area of cellular dysfunction before the occurrence of structural changes (1–3), and perfusion-weighted imaging shows areas of hemodynamic alterations (4, 5), which often exceed the area of abnormal diffusion. The mismatch between diffusion- and perfusion-weighted imaging abnormalities may represent the area of ischemic penumbra (6, 7), which is the target of early treatment. However, MR evaluation of patients with acute stroke should also include conventional sequences, such as MR angiography and contrast-enhanced T1-weighted imaging, because they may contribute to the understanding of the underlying pathophysiologic mechanisms of ischemic stroke.
The role of MR angiography during the early phase of ischemia has been emphasized by recent articles that describe a good correlation between middle cerebral artery (MCA) occlusion and larger areas of both diffusion- and perfusion-weighted imaging mismatch and final lesion size (8, 9). MCA occlusion is a frequent finding in association with early stroke. The evolution of tissue damage is determined, at least in part, by the presence of good collateral circulation, as previously reported in a study on catheter angiography performed during the first 6 hr after stroke (10).
Contrast-enhanced T1-weighted MR images may show vascular enhancement in the territory of cerebral ischemia, a sign that has been found to correlate with angiographic evidence of slow flow in the leptomeningeal collaterals (11). It has been hypothesized that vascular enhancement in association with acute stroke indicates a state of impaired cerebral hemodynamics (11, 12).
Our hypothesis was that because vascular enhancement may be correlated to early development of collateral circulation after MCA occlusion, it is associated with compensatory perfusion changes in the MCA superficial territory, which may influence the final tissue outcome. Therefore, the aim of our work was to investigate the occurrence of vascular enhancement during the first hours after stroke and its relationship to hemodynamic alterations, the status of intracranial vessels, and clinical and tissue outcomes.
Methods
Patients
We studied 15 patients (10 men and five women; age range, 64–84 years; mean age, 73 ± 9 years) with acute ischemic stroke in the MCA territory within 8 hr of symptom onset. Exclusion criteria were hemorrhage revealed by CT or MR imaging, contraindications to MR imaging study, time between stroke onset and MR imaging study longer than 8 hr, and ischemia in other vascular territories. Patients enrolled in stroke therapy trials were not excluded.
Clinical severity was evaluated by means of the National Institutes of Health Stroke Scale at entry, immediately before the MR imaging study, and a week later. Informed consent was obtained from patients who were enrolled in the study and/or from their relatives.
MR Imaging
MR imaging studies were conducted during the acute phase (<8 hr since onset; mean, 4.4 ± 2 hr; range, 1–8 hr) and approximately 1 week later (mean, 6 ± 1.5 days; range, 5–8 days) using a 1.5-T echo-planar imager (Gyroscan, Philips, NT 2000). The sequences used during the acute phase comprised T2-weighted fluid-attenuated inversion recovery (FLAIR) imaging, diffusion-weighted imaging, unenhanced T1-weighted MR imaging, MR angiography, perfusion-weighted imaging, and contrast-enhanced T1-weighted MR imaging, all on the transverse plane, for a total imaging time of 20 min. The sequences used during the postacute phase comprised T2-weighted FLAIR imaging, diffusion-weighted imaging, T1-weighted MR imaging, and MR angiography. With this study, we evaluated diffusion-weighted images, perfusion-weighted images, MR angiograms, and contrast-enhanced T1-weighted MR images obtained during the acute phase and T2-weighted FLAIR images and MR angiograms obtained during the post-acute phase.
Diffusion-weighted Imaging
Diffusion-weighted imaging was acquired using a multisection, single-shot spin-echo echo-planar imaging sequence. The time acquisition was 36 s, and the following parameters were used: 6000/34 (TR/TE); flip angle, 90 degrees; echo-planar imaging factor, 63; b = 0 and 1000; three orthogonal directions; number of sections, 18; section thickness, 7 mm; section gap, 0 mm; matrix, 128 × 128; field of view, 240 mm, 80%.
Perfusion-weighted Imaging
Perfusion-weighted images were obtained using an echo-planar gradient-echo imaging technique before, during, and after the rapid passage of a bolus of IV administered contrast agent (0.2 mmol/kg gadolinium diethylenetriamine penta-acetic acid). Perfusion-weighted imaging was performed with the same imaging matrix, field of view, section thickness, and intersection gap as were used for diffusion-weighted imaging, except with 442/30. Forty images of 12 sections were dynamically acquired with a time resolution of 1.8 s per image. The 12 sections corresponded to 12 of the 18 diffusion-weighted imaging sections, including that of the acute lesion.
MR Angiography
MR angiograms were obtained using a 3D time-of-flight technique, with acquisition of 60 transverse sections (section thickness, 1 mm; section gap, 0) covering the region of the circle of Willis; a 256 × 256 matrix was used. Images were processed with a maximum intensity projection algorithm, with interpolation between sections. Twelve projections were reconstructed along two orthogonal axes.
Data Analysis
Two neuroradiologists who were blinded to the clinical data and the lesion as revealed by MR imaging examined both the source MR angiograms and the reconstructed MR angiograms and indicated the main intracranial artery abnormalities. For the purpose of this study, the patients were divided into groups according to either MCA stem occlusion or patency.
Vascular enhancement was defined as the presence of hyperintense thin vessels at the cortical level on the affected side on T1-weighted contrast-enhanced MR images. All the cerebral sections, from the skull base to the convexity, were inspected for detection of vascular enhancement. Vascular enhancement was classified as absent, mild, or marked. Marked vascular enhancement was defined as the appearance of at least three vessels on at least two sections.
Lesion volume was measured on early isotropic diffusion-weighted images by manually outlining the hyperintense area on all the sections in which it was evident (b = 1000). The areas of diffusion-weighted imaging hyperintensity were then summed, and the value obtained was multiplied by the section thickness. The same method was applied to measure the final lesion volume on T2-weighted FLAIR images obtained 1 week later.
Perfusion images were post processed to generate maps of relevant hemodynamic parameters. Relative cerebral blood volume (rCBV) maps were calculated by integrating the gamma fitted ΔR2* curve; an index of the vascular transit time was derived from the first moment of the curve (mean transit time [MTT]) and from the time between the beginning of the experiment and the maximum signal drop due to contrast passage into the brain (time to peak [TTP]) (13).
The volume of abnormal hemodynamics was calculated by including the region with increased TTP by using the same method as that used for diffusion-weighted and FLAIR images. TTP maps were chosen for this analysis because the hyperintense area was more evident and the contour between the hyperintense area and surrounding tissue was sharper on TTP maps than on the other perfusion-weighted imaging maps. The regions outlined on TTP maps never included the area of abnormality shown on the diffusion-weighted images.
On the two sections showing maximal TTP abnormalities, the outlined regions were mirrored onto the unaffected hemisphere to calculate an index of asymmetry in rCBV and MTT as follows: affected side − unaffected side × 100/unaffected side. The two values obtained for each perfusion-weighted imaging parameter were then averaged.
Results
The clinical and MR findings of the 15 patients enrolled in this study are summarized in Table 1. The perfusion-weighted imaging maps of three patients (patients 11, 14, and 15) were not reliable because of movement artifacts during acquisition. Follow-up MR images were not obtained from these same three patients because the patients died.
TABLE 1:
Clinical and MR data from 15 patients studied within 8 hr since stroke onset
Early MR angiography showed occlusion of the MCA stem in nine patients and a patent MCA stem in each of the remaining six. MR angiography performed 1 week later showed the recanalization of the MCA in all the cases with earlier defects.
Volumes of lesions revealed by early diffusion-weighted imaging and volumes of early altered hemodynamics were significantly larger in patients with MCA stem occlusion than in patients with MCA stem patency (Table 2). Both rCBV and MTT asymmetries were larger in patients with MCA occlusion, but statistical significance was not reached because of the large variability of these two hemodynamic parameters in both the patent and occluded MCA groups (Table 2). The final lesion volume (based on FLAIR imaging performed at 1 week) was significantly larger, and National Institutes of Health Stroke Scale scores (both at admission and 1 week later) were higher in patients with MCA occlusion than in patients with MCA patency (Table 2).
TABLE 2:
Comparison between patients either with or without MCA stem occlusion
Vascular enhancement was observed both in patients with MCA occlusion and in those with normal results of their MR angiography. The degree of vascular enhancement was marked in seven of the nine patients with MCA occlusion and absent in the remaining two. In patients with MCA patency, vascular enhancement was marked in only one case, mild in four, and absent in one.
Two patients (patients 14 and 15) with MCA occlusion and without vascular enhancement had very large lesions (mean, 352 mm3) revealed by early diffusion-weighted imaging, covering the entire territory of the MCA, and a poor prognosis (both patients died within 1 week) (Table 1).
Of the seven patients with MCA stem occlusion and marked vascular enhancement, two (patients 8 and 12) had damage confined to the basal ganglia as shown by both the early (diffusion-weighted) and late (FLAIR) MR images (Fig 1). In one patient (patient 9), damage revealed by early diffusion-weighted imaging was prevalently localized in the deep MCA territory and then enlarged to include the superficial MCA territory. In four other patients (patients 7, 10, 11, and 13) the parenchymal damage affected part of the superficial MCA territory. The extent of lesion increase in these patients as revealed by follow-up MR imaging varied greatly (Table 1).
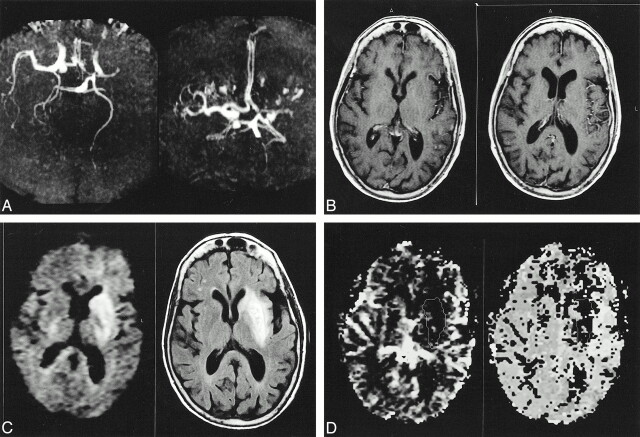
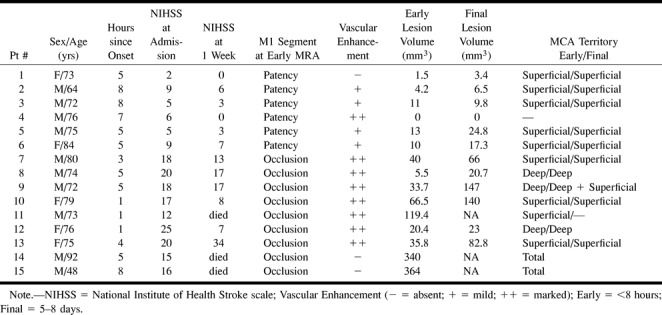
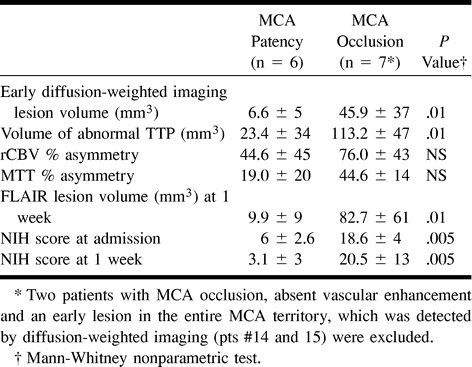
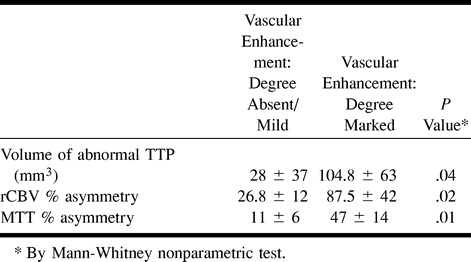
fig 1.
MR images of patient 12, who was studied 1 hr after the onset of stroke.
A, MR angiogram (43/2.3/1 [TR/TE/excitations]) shows left MCA occlusion, mild caliber reduction of the supraclinoid tract of the left internal carotid artery with respect to the contralateral one, asymmetry in the A1 segments (right > left) with good filling of both the anterior cerebral arteries (A2 tracts), and left posterior cerebral artery filling out farther than the contralateral one.
B, Contrast-enhanced T1-weighted images (560/14/2) show marked vascular enhancement of the thin vessels in the left superficial frontotemporal region.
C, Diffusion-weighted image (left) (6000/64/1) shows early damage in the left deep MCA territory. The lesion extent is substantially unchanged on the follow-up MR image (right) (FLAIR image obtained 1 week later [6000/100/2]).
D, Perfusion-weighted maps show an increase of both rCBV (left) and MTT (right) in the left superficial MCA territory, outside the area of altered diffusion-weighted imaging (outlined). The rCBV and MTT asymmetries were 105% and 54%, respectively.
Only one patient (patient 4) with MCA stem patency showed marked vascular enhancement. For that patient, both early diffusion-weighted and late FLAIR imaging findings were normal. For the other patients with MCA stem patency, vascular enhancement was mild or absent. All these patients had a good prognosis in terms of both clinical and tissue outcomes.
Perfusion-weighted imaging findings in relation to the degree of vascular enhancement are shown in Table 3. Patients with marked vascular enhancement had significantly larger regions of altered hemodynamics, as expressed by the volume of increased TTP. They also had significantly higher asymmetries in both rCBV and MTT because of increased values in the affected hemisphere. Both rCBV and MTT were greatly increased (134% and 58%, respectively) for patient 4, who had marked vascular enhancement despite MCA patency.
TABLE 3:
Hemodynamic findings in patients with either absent/mild or marked vascular enhancement on postcontrast T1-weight~ed images in the superficial territory of the MCA
Discussion
In this study of acute ischemic stroke, we focused on two main findings: the presence of MCA occlusion and the presence of vascular enhancement. We investigated whether these two findings are related and, if so, how they predict clinical and tissue outcomes.
Although unenhanced MR angiography may have some limits in diagnostic accuracy because of relative insensitivity to slow flow, an excellent correlation between the findings of MR angiography and those of conventional angiography has been reported for detection of intracranial vascular alterations (14). Furthermore, conventional angiography performed during the first 6 hr after the onset of stroke reveals intracranial arterial occlusion in the majority of patients (15), whereas intracranial arterial stenosis is a rare event.
Patients with MCA stem occlusion had more severe clinical presentations and larger areas of tissue damage at the time of early diffusion-weighted imaging than did patients with MCA stem patency. They also presented larger areas of altered hemodynamics and had a poorer clinical and tissue outcomes. These results are in agreement with those of previous MR imaging studies, which reported that patients with stroke and MCA stem occlusion had more extended areas of ischemic penumbra and a final infarct size larger than that of the lesion revealed by initial diffusion-weighted imaging (8, 9).
Additional information can be obtained by performing contrast-enhanced T1-weighted MR imaging. Regarding vascular enhancement, our data highlighted two main points: cortical areas with marked vascular enhancement are not necessarily destined to evolve toward infarct, and both rCBV and MTT are increased in cortical areas showing vascular enhancement.
Sato et al (16) described abnormally contrast-enhanced curvilinear structures in the evolving area of cerebral ischemia/infarction in eight patients within 26 hr of ischemic stroke. They interpreted the abnormal enhancement as cortical arterial vessels of markedly slowed circulation in areas with underlying brain injury. Because the MR signal of vascular enhancement is due to slow flow in leptomeningeal collaterals, as previously reported on the basis of angiographic data (11, 12), the presence of vascular enhancement in patients with MCA occlusion indicates the existence of collateral circulation.
In our sample, the two patients presenting with MCA occlusion and no vascular enhancement exhibited impaired diffusion in the complete MCA territory and had a poor prognosis. This finding is in agreement with those of a previous study, which highlighted the role played by early collateral blood supply after MCA occlusion in limiting parenchymal brain damage (10). In that study, the absence of efficient collateral blood circulation was associated with infarction in the complete MCA territory in the majority of patients.
We observed that marked vascular enhancement in patients with MCA occlusion was significantly associated with increased CBV and MTT in the superficial MCA territory (Fig 1). This finding indicates a compensatory attempt to balance the decreased perfusion pressure by means of vasodilation of collateral vessels. We found vascular enhancement and CBV increase outside the area of diffusion-weighted imaging hyperintensity. This pattern represents the area of diffusion/perfusion-weighted imaging mismatch that is at risk of infarction (7, 17, 18). The transition from ischemic tissue to irreversible damage is unpredictable and depends on a number of factors, which include the level of ischemia and the time (19). The compensatory collateralization and CBV increase are time-dependent and are able to preserve tissue from infarction only during the early phase. No patient with MCA occlusion, marked vascular enhancement, and increased CBV had a complete MCA infarction revealed by the first MR imaging study. The evolution of the infarct varied in these patients. Follow-up MR imaging revealed that cerebral areas belonging to the superficial MCA territory were spared in the two patients with initial damage in the basal ganglia and MCA occlusion proximal to the origin of the lenticulostriate arteries. Varying amounts of cerebral tissue supplied by MCA distal branches were affected in the other five patients. Final lesion size varied from 20 to 147 mm3. This different evolution is probably also determined by the duration of the MCA occlusion. Our data indicate that MCA recanalization was present at 1 week in all the patients, but we cannot know exactly when it occurred. Studies using MR angiography and/or transcranial Doppler sonography aimed at monitoring the time of reperfusion could be helpful to elucidate the relationship between the duration of MCA occlusion and the final size of the ischemic infarct.
Interestingly, increased CBV was associated with marked vascular enhancement in a patient with MCA stem patency and normal diffusion-weighted imaging findings, despite the presence of focal neurologic symptoms. We can hypothesize that an early spontaneous MCA recanalization had occurred in this patient before the MR imaging study was performed. Hemodynamic response to the arterial occlusion, such as the CBV increase due to vasodilation, may persist during the postocclusion period. Experimental studies have shown hemodynamic alterations associated with MCA occlusion, which persisted for some time after reperfusion (20, 21).
The results of this and numerous previous studies (2, 4, 5, 7–9, 22–24) indicate that MR imaging may contribute to our understanding of the pathophysiologic mechanisms of ischemic stroke and may help guide the therapeutic approach. In particular, the role of MR angiography in indicating the real target of thrombolytic therapy and in predicting both the tissue and clinical outcome is unequivocal. As previously observed (10), the final damage size depends on the presence of sufficient leptomeningeal collaterals. Arterial main stem occlusions can easily be shown by MR angiography, whereas the occlusion of distal branches may be overlooked and collateral blood supply cannot be detected.
Although vascular enhancement is not a highly specific finding because it may be observed both in the presence or absence of arterial occlusion, it assumes a great significance in patients with MCA occlusion by indicating the presence of good collateral circulation. It provides a potentially alternative way of documenting an at least partially preserved perfusion when perfusion-weighted images are not available. Studies with a larger patient base are warranted to confirm our results by increasing the specificity of these MR imaging findings in patients with acute stroke.
Footnotes
Address reprint requests to Patrizia Pantano, MD, Department of Neurological Sciences, Viale dell'Università, 30, I-00185 Rome, Italy.
References
- 1.Moseley M, Kucharczyk J, Mintorovitch J, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol 1990;11:423-429 [PMC free article] [PubMed] [Google Scholar]
- 2.Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol 1995;37:231-241 [DOI] [PubMed] [Google Scholar]
- 3.Lutsep H, Albers G, DeCrespigny A, Kamat G, Marks M, Mosely M. Clinical utility of diffusion-weighted magnetic resonance imaging in the assessment of ischemic stroke. Ann Neurol 1997;41:574-580 [DOI] [PubMed] [Google Scholar]
- 4.Röther J, Gückel F, Neff W, Schwartz A, Hennerici M. Assessment of regional cerebral blood volume in acute human stroke by use of single-slice dynamic susceptibility contrast-enhanced magnetic resonance imaging. Stroke 1996;27:1088-1093 [DOI] [PubMed] [Google Scholar]
- 5.Sorensen A, Copen W, Ostergaard L, et al. Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative blood flow and mean tissue transit time. Radiology 1999;210:519-527 [DOI] [PubMed] [Google Scholar]
- 6.Karonen JO, Vanninen R, Liu Y, et al. Combined diffusion and perfusion MRI with correlation to single-photon emission CT in acute ischemic stroke: ischemic penumbra predicts infarct growth. Stroke 1999;30:1583-1590 [DOI] [PubMed] [Google Scholar]
- 7.Neumann-Haefelin T, Wittsack HJ, Wenserski F, et al. Diffusion- and pefusion-weighted MRI: the DWI/PWI mismatch region in acute stroke. Stroke 1999;30:1591-1597 [DOI] [PubMed] [Google Scholar]
- 8.Rordorf G, Koroshetz W, Copen W, et al. Regional ischemia and ischemic injury in patients with acute middle cerebral artery stroke as defined by early diffusion-weighted and perfusion-weighted MRI. Stroke 1998;29:939-943 [DOI] [PubMed] [Google Scholar]
- 9.Barber P, Davis SM, Darby DG, et al. Absent middle cerebral artery flow predicts the presence and evolution of the ischemic penumbra. Neurology 1999;52:1125-1132 [DOI] [PubMed] [Google Scholar]
- 10.Bozzao L, Fantozzi LM, Bastianello S, Bozzao A, Fieschi C. Early collateral blood supply and late parenchymal brain damage in patients with middle cerebral artery occlusion. Stroke 1989;20:735-740 [DOI] [PubMed] [Google Scholar]
- 11.Mueller D, Yuh W, Fisher D, Chandran K, Crain M, Kim Y. Arterial enhancement in acute cerebral ischemia: clinical and angiographic correlation. AJNR Am J Neuroradiol 1993;14:661-668 [PMC free article] [PubMed] [Google Scholar]
- 12.Essig M, von Kummer R, Egelhof T, Winter R, Sartor K. Vascular MR contrast enhancement in cerebrovascular disease. AJNR Am J Neuroradiol 1996;17:887-894 [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen B, Belliveau J, Buchbinder B, et al. Contrast agents and cerebral hemodynamics. Magn Reson Med 1991;19:285-292 [DOI] [PubMed] [Google Scholar]
- 14.Heiserman J, Drayer BP, Keller PJ, Fram EK. Intracranial vascular stenosis and occlusion: evaluation of three-dimensional time-of-flight MR angiography. Radiology 1992;185:667-673 [DOI] [PubMed] [Google Scholar]
- 15.Fieschi C, Argentino C, Lenzi G, Sacchetti M, Toni D, Bozzao L. Clinical and instrumental evaluation of patients with ischemic stroke within the first six hours. J Neurol Sci 1989;91:311-322 [DOI] [PubMed] [Google Scholar]
- 16.Sato A, Takahashi S, Soma Y, et al. Cerebral infarction: early detection by means of contrast-enhanced cerebral arteries at MR imaging. Radiology 1991;178:433-439 [DOI] [PubMed] [Google Scholar]
- 17.Baird AE, Benfield A, Schlaug G, et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol 1997;41:581-589 [DOI] [PubMed] [Google Scholar]
- 18.Beaulieu C, de Crespigny A, Tong D, Moseley ME, Albers G, Marks M. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann Neurol 1999;46:568-578 [DOI] [PubMed] [Google Scholar]
- 19.Astrup J, Siesjo BK, Symon L. Threshold in cerebral ischemia: the ischemic penumbra. Stroke 1981;12:723-725 [DOI] [PubMed] [Google Scholar]
- 20.Caramia F, Huang Z, Hamberg L, et al. Mismatch between cerebral blood volume and flow index during transient focal ischemia studied with MRI and Gd-BOPTA. Magn Reson Imaging 1998;16:97-103 [DOI] [PubMed] [Google Scholar]
- 21.van Lookeren Campagne M, Thomas GR, Thrbodeaux H, et al. Secondary reduction in the apparent diffusion coefficient of water, increase in cerebral blood volume, and delayed neuronal death after middle cerebral artery occlusion and early reperfusion in the rat. J Cereb Blood Flow Metab 1999;19:1354-1364 [DOI] [PubMed] [Google Scholar]
- 22.Warach S, Dashe J, Edelman R. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging: a preliminary analysis. J Cereb Blood Flow Metab 1996;16:53-59 [DOI] [PubMed] [Google Scholar]
- 23.Lovblad KO, Laubach HJ, Baird AE, et al. Clinical experience with diffusion-weighted MR in patients with acute stroke. AJNR Am J Neuroradiol 1998;19:1061-1066 [PMC free article] [PubMed] [Google Scholar]
- 24.Kidwell CS, Alger JR, Di Salle F, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke 1999;30:1174-11801 [DOI] [PubMed] [Google Scholar]