Abstract
Summary: In a patient with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS), both T2- and diffusion-weighted MR imaging revealed lesions as hyperintense areas in the occipital lobes early after strokelike episodes. In these lesions, no significant reduction in apparent diffusion coefficient was noted. Apparent diffusion coefficient mapping may help to differentiate strokelike episodes in MELAS from acute ischemic stroke. The strokelike episodes may be related to vasogenic edema and hyperperfusion, which were suggested by our single-photon emission CT and MR imaging studies.
Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes (MELAS) is a well-known clinical syndrome of inherited mitochondrial disorders (1), and strokelike episodes occur in more than 90% of patients with MELAS (2). Although diffusion-weighted MR imaging is a powerful tool for detecting early ischemic brain lesions with a significant reduction in apparent diffusion coefficient (ADC), the findings of diffusion-weighted imaging and ADC maps early after strokelike episodes in cases of MELAS have not been reported to date. We herein report the findings of diffusion-weighted imaging and ADC maps of a patient with a confirmed diagnosis of MELAS who exhibited strokelike episodes.
Case Report
The patient was a 41-year-old man who had no family history of neurologic disease. He had experienced a generalized convulsion at age 3 years and was diagnosed as having hypertension and hyperlipidemia at age 35 years.
The patient was first admitted to our hospital on April 10, 1995, because of acute onset of blindness and generalized convulsion. Eight days after the episode, MR imaging was performed using a Siemens MAGNETOM 1.5-T spin-echo system. T2-weighted (1800/110 [TR/TE]) imaging revealed small hyperintense lesions located in the bilateral occipital cortices. Patchy and punctate enhancement was noted on the T1-weighted (500/20) images (Fig 1A and B) after the administration of gadolinium-diethylenetriamine pentaacedic acid. Symptoms disappeared within 2 weeks, and no lesion was revealed by MR imaging performed 2 months later (Fig 1C and D). Mitochondrial DNA analysis of peripheral blood leukocyte samples yielded an A → G point mutation in the transfer RNALeu gene at base pair 3243, thereby confirming the clinical diagnosis of MELAS. The patient was discharged from the hospital without neurologic deficits on June 18, 1995.
fig 1.
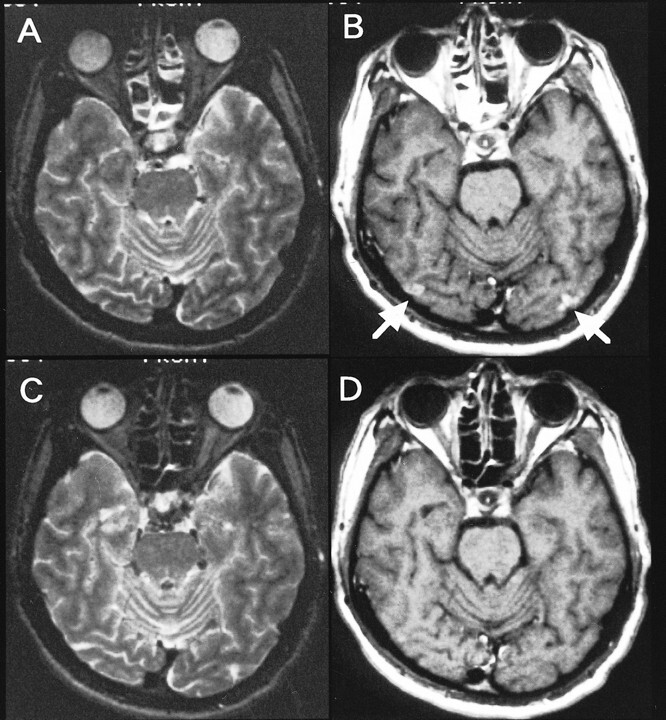
A, Axial T2-weighted image obtained at the time of initial hospitalization (8 days after the onset of symptoms).
B, Contrast-enhanced T1-weighted image reveals small hyperintense lesions with patchy and punctate enhancement in the bilateral occipital cortices.
C and D, Follow-up images obtained 2 months later show resolution of the lesions.
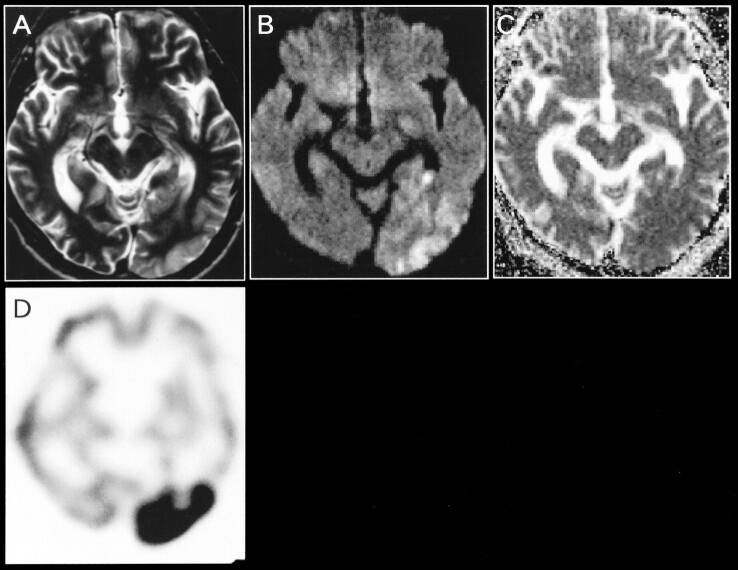
On October 7, 1997, the patient was again admitted to our hospital because of pulsating headache and right homonymous hemianopia. He was examined the next day by use of a Siemens MAGNETOM Vision 1.5-T MR unit, echo-planar imaging system. Diffusion-weighted imaging was performed using a multisection, single-shot, spin-echo echo-planar imaging sequence. Diffusion gradients were applied in each of the x, y, and z directions with two b values (0 and 1000 s/mm2). The ADC maps were created by signals obtained from images with two b values according to the method previously described (3). The mean ADC was calculated as one-third (ADCx + ADCy + ADCz). T2- and diffusion-weighted imaging revealed an extensive hyperintense lesion involving cortex and subcortical white matter in the left occipital lobe (Fig 2A and B). No contrast enhancement was observed. The mean ADC (±SD) values were 0.92 (±0.07) 10−3mm2/s for the left occipital lesion and 0.82 (±0.11) 10−3mm2/s for the contralateral control region (not significantly different) (Fig 2C). Single-photon emission CT (SPECT) with 99mTc-hexamethylpropyleneamine oxime (99mTc-HMPAO) on day 3 showed remarkably increased tracer uptake in the left occipital lobe (Fig 2D) that corresponded to the abnormality revealed by MR imaging. Right homonymous hemianopia recovered on day 5. Two weeks later, no MR abnormalities were detected in the left occipital cortex, with the exception of mild localized atrophy. The patient was discharged on November 13, 1997.
fig 2.
A, Axial T2-weighted image obtained at the time of the second hospitalization (2 days after the onset of symptoms).
B, Diffusion-weighted image shows an extensive hyperintense lesion involving the cortex and subcortical white matter in the left occipital lobe.
C, Corresponding ADC map reveals no obvious abnormalities.
D, 99mTc-HMPAO SPECT scan obtained on day 3 shows a remarkable increase in tracer accumulation in the left occipital lobe. Follow-up images obtained 2 weeks later (not shown) indicated the resolution of the left occipital abnormality with residual localized atrophy.
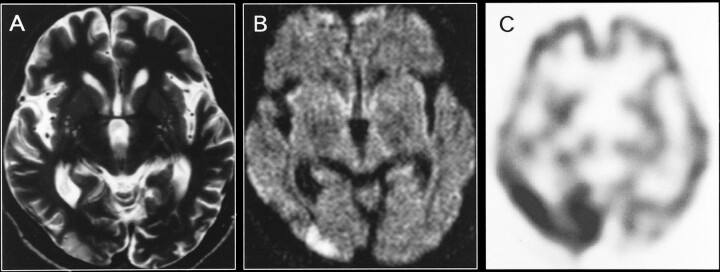
The patient was readmitted on July 8, 1998, because of left homonymous hemianopia. On day 2 after the symptom onset, T2- and diffusion-weighted imaging revealed a cortical hyperintense lesion in the right occipital lobe (Fig 3A and B) but no contrast enhancement. ADC (±SD) values for the right occipital lesion and contralateral region were 1.18 (±0.09) 10−3mm2/s and 0.92 (±0.22) 10−3mm2/s, respectively. A SPECT study, performed on the same day, showed increased 99mTc-HMPAO accumulation in the right occipital cortex (Fig 3C). Hemianopia completely recovered by day 4. Follow-up MR imaging performed 2 weeks later showed a resolution of the right occipital abnormality. The 99mTc-HMPAO SPECT study conducted 16 days after the onset of symptoms showed normalization in the right occipital cortex. The patient was discharged on July 31, 1998.
fig 3.
A, Axial T2-weighted image obtained at the time of the third hospitalization (2 days after the onset of symptoms).
B, Diffusion-weighted image reveals a cortical hyperintense lesion in the right occipital lobe.
C, 99mTc-HMPAO SPECT scan obtained on the same day shows a remarkable increase in tracer accumulation in the right occipital cortex. Follow-up MR images obtained 2 weeks later (not shown) revealed resolution of the right occipital abnormality, and SPECT scans obtained 16 days after the onset of symptoms (not shown) showed a normal accumulation in the right occipital cortex.
Discussion
Diffusion-weighted imaging can rapidly detect and localize focal ischemic lesions as hyperintense regions within a few minutes after the onset of symptoms (4). A significant ADC decline is observed for several days in cases of acute ischemic lesions (3), which is presumed to be a result of intracellular water accumulation (cytotoxic edema) caused by energy failure. The ADC decline is a phenomenon observed not only in cases of acute ischemic stroke but also in association with various pathologic conditions, such as status epilepticus (5), severe hypoglycemia (6), and cortical spreading depression (7, 8). These ADC declines have been attributed to factors such as intracellular edema, membrane permeability changes, and a significant shrinkage of the extracellular space volume. However, there has been no information regarding diffusion-weighted imaging findings and ADC changes early after strokelike episodes in cases of MELAS.
Although the pathogenesis of strokelike episodes has not been fully elucidated, it may be due to an energy production failure in central nerve cells caused by mitochondrial dysfunction (9). Such an energy production failure would result in significant ADC reduction, as observed in cases of acute ischemic stroke. Nevertheless, a significant ADC reduction was not observed in the present case. The normal ADC value in the brain tissues reported by Schlaug et al (3) was 0.907 × 10−3mm2/s (3), which is comparable with the results reported herein.
Ohama et al (10) stated the importance of another mechanism for strokelike episodes in cases of MELAS (ie, abnormality of small arteries, described as mitochondrial angiopathy). In our case, the hyperintensity on T2-weighted images and scattered enhancement on contrast-enhanced MR images obtained at the time of the first hospitalization may reflect a breakdown of the blood-brain barrier and early development of vasogenic edema. The increased tracer uptake observed on 99mTc-HMPAO SPECT scans also suggested blood-brain–barrier breakdown. Therefore, we think that the findings of SPECT had features similar to those of MR imaging. On the other hand, increased regional cerebral blood flow in the affected areas was reported during the strokelike episodes in cases of MELAS and was presumed to result from vasodilation due to lactic acidosis (11).
Conclusion
The present results indicate that strokelike episodes in cases of MELAS are not only caused by energy failure but are also related to hyperperfusion and vasogenic edema. ADC mapping may help to differentiate between ischemic stroke and strokelike episodes in cases of MELAS early after the onset of symptoms.
Acknowledgments
We thank Dr. Naoaki Yamada (Department of Diagnostic Radiology) for valuable comments regarding the MR imaging studies. This study was supported in part by Research Grants for Cardiovascular Diseases (8C-4, 9A-2, 9A-3, 9A-8) from the Ministry of Health and Welfare of Japan and by Special Coordinating Funds for Promoting Science and Technology (Strategic Promotion System for Brain Science) from the Science and Technology Agency of Japan.
Footnotes
Address reprint requests to Kiminobu Yonemura, MD, Cerebrovascular Division, Department of Medicine, National Cardiovascular Center, 5-7-1 Fujishirodai, Suita, Osaka 565-8565, Japan.
References
- 1.Pavlakis SG, Phillips PC, DiMauro S, De Vino DC, Rowland LP. Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes: a distinctive clinical syndrome. Ann Neurol 1984;16:481-488 [DOI] [PubMed] [Google Scholar]
- 2.Goto Y, Horai S, Matsuoka T, et al. Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS): a correlative study of the clinical features and mitochondrial DNA mutation. Neurology 1992;42:545-550 [DOI] [PubMed] [Google Scholar]
- 3.Schlaug G, Siewert B, Benfield A, Edelman RR, Warach S. Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology 1997;49:113-119 [DOI] [PubMed] [Google Scholar]
- 4.Minematsu K, Li L, Fisher M, Sotak CH, Davis MA, Fiandaca MS. Diffusion-weighted magnetic resonance imaging: rapid and quantitative detection of focal brain ischemia. Neurology 1992;42:235-240 [DOI] [PubMed] [Google Scholar]
- 5.Zhong J, Petroff AC, Prichard JW, Gore JC. Changes in water diffusion and relaxation properties of a rat cerebrum during status epilepticus. Magn Reson Med 1993;30:241-246 [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa Y, Formato JE, Latour LL, et al. Severe transient hypoglycemia causes reversible change in the apparent diffusion coefficient of water. Stroke 1996;27:1648-1656 [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa Y, Latour LL, Formato JE, Sotak CH, Fisher M. Spreading waves of a reduced diffusion coefficient of water in normal and ischemic rat brain. J Cereb Blood Flow Metab 1995;15:179-187 [DOI] [PubMed] [Google Scholar]
- 8.Leao AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol 1944;7:359-390 [DOI] [PubMed] [Google Scholar]
- 9.Barbiroli B, Montagna P, Martinelli P, et al. Defective brain energy metabolism shown by in vivo 31P MR spectroscopy in 28 patients with mitochondrial cytopathies. J Cereb Blood Flow Metab 1993;13:469-474 [DOI] [PubMed] [Google Scholar]
- 10.Ohama E, Ohara S, Ikuta F, Tanaka K, Nishizawa M, Miyatake T. Mitochondrial angiopathy in cerebral blood vessels of mitochondrial encephalomyopathy. Acta Neuropathol (Berl) 1987;74:226-233 [DOI] [PubMed] [Google Scholar]
- 11.Ooiwa Y, Uematsu Y, Terada T, et al. Cerebral blood flow in mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Stroke 1993;24:304-309 [DOI] [PubMed] [Google Scholar]