Abstract
Summary: A laboratory-based study of the physical and performance characteristics of a new 0.25-mm-thin microangioscope was performed. The microangioscope tested was compatible with currently available microcatheters, but its tip was considerably stiff and of limited radiopacity. Poor image quality and difficult image interpretation were further drawbacks. Intensive efforts are directed at addressing current limitations and testing further innovations that could pave the way for future performance in neurovascular endoscopy.
A new prototype 0.25-mm microangioscope (an ultrathin vascular endoscope capable of passage through angiographic microcatheters) has been manufactured recently by Mitsubishi Cable Industries, Ltd., Tokyo, Japan. The size of this new microangioscope now intimates the possibility of using it in the cerebral and craniofacial vascular systems, with potential acquisition of in vivo images hitherto unavailable. The aim of this experimental study was to investigate the feasibility of extending this technology to imaging within the intracranial circulation by the initial bench-top and in vivo laboratory testing of the physical and performance characteristics of this ultrathin microangioscope and its suitability for potential use in interventional neuroradiologic practice.
Methods and Results
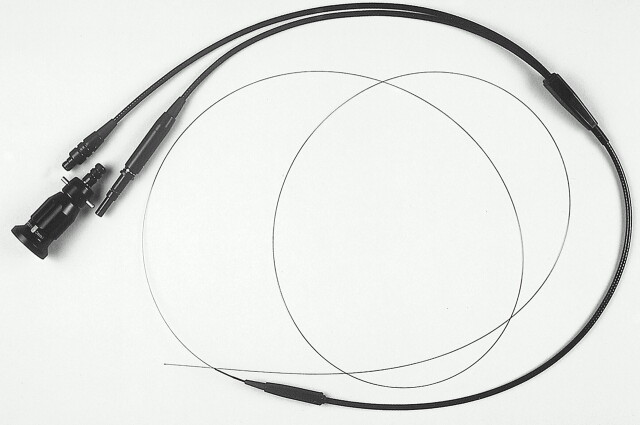
The microangioscope under evaluation (Figs 1 and 2) is a straight-tipped, two-function (image transmission and illumination), single-lumen angioscope of 0.25-mm diameter and 150-cm length, with six 18-μm-diameter glass light guide fibers and pure silica image bundles yielding 2000 pixels (each pixel, 2.8 μm), encased in a polyimide outer jacket. It has a 55-degree angle of view, and a 1- to 10-mm depth of field. An objective self-focusing Selfoc rod lens (Nihon Sheet Glass Co., Ltd., Tokyo) focuses the image of the object to the distal end of the image guide. Eyepiece lenses offer magnification of the image that vary from ×31 to ×83. The pure silica imaging fibers used in this endoscope are superior to conventional glass fibers regarding fineness, durability, and cost; pure silica image fibers are “fuse-bundled” and used without further processing as an image guide. On the other hand, multicomponent glass image bundles that are made by the “acid leaching” method are flexible but require a protective tube and other parts that impose limits on diameter fineness. Furthermore, this endoscope benefits from a maximum density regular alignment of fibers of identical diameter. This differs from other pure silica image guide instruments, which generally contain a random arrangement of fibers of different diameters that yield an inferior picture quality (1).
fig 1.
New 0.25-mm microangioscope and eyepiece
A 250-watt metal halide arc light source (Medicam 900 Light Source; MP Video, Inc., Medway, MA) with a high-intensity output lamp was used to provide illumination for all angioscopic investigations. Images obtained were relayed through a video endocoupler to a light-sensitive video camera (Medicam 900 DigiCon Camera; MP Video, Inc.) containing a charged-coupled device microlens and circuitry that converts the image into an electrical signal. When connected to a camera control unit, the electrical signal is converted into a video signal for display on a television monitor (Sony Trinitron PVM-1343MD; Sony Corporation, Tokyo, Japan) or for recording on a video recorder (JVC SR-S36OU; JVC, Tokyo, Japan) or a video printer (Sony Mavigraph UP-1200, Sony Corporation). The television camera and monitor are required because the fiberoptic imaging bundle is too small for the unaided eye to see the live image without magnification (2).
For in vivo use, a specially constructed angioscopic fluid irrigation system (Angiopump; Olympus Corp, Lake Success, NY) was used to remove blood from the field of view with a carefully controlled flow of pressurized clear irrigating solution. The Angiopump is a peristaltic roller pump that provides a pulsatile constant volume irrigation of normal saline or lactated Ringer's solution through the infusion lumen of the balloon catheter that surrounds the microangioscope. The pump's tubing was connected to the infusion lumen of the balloon catheter with a standard three-way valve. Irrigation was started in high flow mode with 400 mL/min to flush away the blood completely. Low-flow mode with 50 to 200 mL/min was sufficient to maintain clarity of intravascular vision. The change of flow was attained with a foot pedal control mechanism. The use of this fluid irrigation system has been shown previously to result in shorter visualization times, decreased irrigation volumes, and improvement in image quality and diagnosis (3).
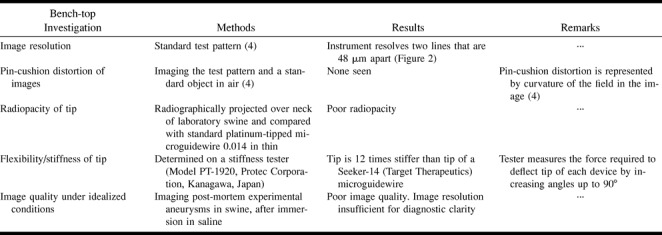
Bench-top and in vivo laboratory investigations of the microangioscope were performed. These studies and the results obtained are summarized in Tables 1 and 2. All animal experimentation was conducted in accordance with policies set by the local University Chancellor's Animal Research Committee and National Institutes of Health guidelines. Five Red Duroc swine of mixed sex and weighing 30 to 40 kg each were maintained on a standard laboratory diet and used in this research. After an overnight fast, each swine was premedicated with intramuscular 20 mg/kg ketamine and intramuscular 2 mg/kg xylazine. General anesthesia was maintained with mechanical ventilation and inhalation of 1% to 2% halothane after endotracheal intubation.
TABLE 1:
Summary of bench-top investigations of the microangioscope and results obtained
Discussion
The general characteristics of video angioscopy that make it attractive are as follows (5): 1) it delivers a 3D magnified color image of the vessel lumen and surface topography of the intima, with ample clarity for clinical decision making; 2) multiple/repeated examinations of various areas can be made in a short period of time; 3) no potential toxic or allergic substances are used; 4) the entire examination can be recorded and stored for later retrieval; and 5) the technique can be used without compromising the sterile field.
At present, the limitations of angioscopy include its ability to examine only vessels of appropriate size and accessibility and the lack of information concerning the more distal vascular tree. In this respect, angioscopy cannot expect to replace angiography (5). Angioscopy does, however, have the potential for selected future intracranial applications as an adjunct to angiography, as is the case in peripheral and coronary vessels. To image intracranial arteries successfully percutaneously (as opposed to performance of intraoperative angioscopy through an open craniotomy), a number of technical hurdles must be overcome (2). The angioscope with or without a surrounding catheter has to be small and flexible enough to be advanced atraumatically. Once at the target site, a blood-free field must be created and maintained without local and distal neuronal ischemia or embolic complications. Finally, the operator must be able to control the distal tip of the angioscope so that a complete visual examination of the vessel lumen can be obtained in tortuous vessels.
A serious concern that would arise when infusing fluid intraarterially at high flow rates is that excessively high intraarterial pressures may be generated that could damage either the intimal lining or the inner layer of the arterial wall, even to the extent of complete rupture (6). In the present study, we examined this issue further by direct measurements of intraaneurysmal pressures during angioscopic fluid irrigation in swine lateral aneurysm models. There were no significant increases in pressures during this maneuver. This may represent an additional point of reassurance regarding the potential safety of future intracranial angioscopy in that it may not predispose to barotrauma and rupture of fragile intracranial aneurysms. The almost constant presence of side branches in relation to aneurysms would also aid in dissipation of the intravascular/intraaneurysmal pressure generated during angioscopic irrigation. However, caution is necessary before clinical application for the following reasons. First, lateral aneurysms are not truly representative of intracranial aneurysms and, therefore, it is possible that pressure rises might be higher within more hydrodynamically realistic bifurcation or terminal aneurysms. Second, the pressure rises might be higher at the time of angioscopic irrigation into a relatively restricted outflow tract (6) (eg, in the presence of vasospasm consequent to aneurysmal subarachnoid hemorrhage). Third, extra care may have to be exercised in future angioscopic irrigation in the presence of a recently ruptured aneurysm that may have its tear plugged with fresh delicate thrombus. These factors require further investigation in appropriate experimental models before clinical extrapolation.
In conclusion, the image quality of the evaluated 0.25-mm microangioscope was deemed unsatisfactory for widespread applications. Although the tip of this instrument is too stiff for unprotected use in distal intracranial arteries, it might possibly have limited applications (if high quality of the image is not a priority) in cervicofacial and proximal intracranial arteries by positioning it within microcatheters commonly used in interventional neuroradiologic procedures in such a manner that its tip does not protrude beyond that of a surrounding microcatheter. A more ideal instrument would possess better optics, improved steerability, angular tip movement, greater radiopacity of its tip for fluoroscopic visualization, and a considerably less stiff tip. Achievement of these goals should be the subject of intensive efforts if interventional neuroradiologic use is contemplated. Further experimental evaluations also are necessary to assess the full capabilities and safety of this instrument when imaging appropriate animal models of intracranial vasculopathies before future clinical application. Despite several current limitations, percutaneous angioscopy of the cerebral and craniofacial vasculature could become a realistic and valid proposition in the not-too-distant future. Although the clinical applications and significance of angioscopic images remain to be determined, it is suspected that the use of angioscopy as an exciting and innovative imaging tool could become an integral part of future investigations of patients with neurovascular disease, the tailoring of endovascular therapy, and the management of complications after treatment. The new prototype microangioscope presented in this study represents an initial step in this direction and, with continued modifications and improvements, appears promising as an addition to the armamentarium of interventional neuroradiologists in their quest to perform clinical percutaneous cerebrovascular endoscopy.
TABLE 2:
Summary of in vivo investigations of the microangioscope and results obtained
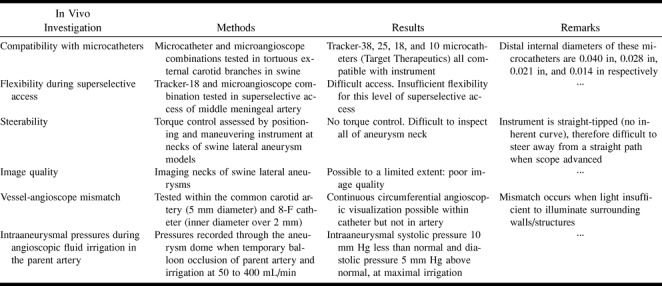
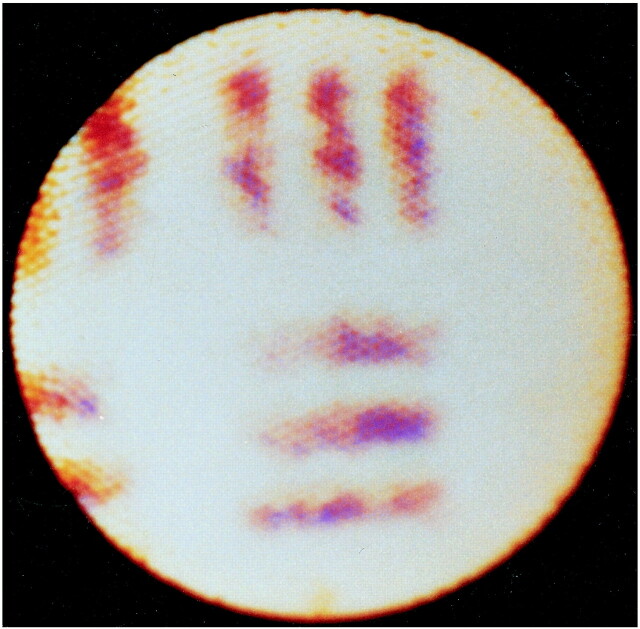
fig 2.
Image of a test pattern showing clear separation of 21 lines per millimeter. The spatial resolution of the instrument was determined in air at a constant distance of 3 mm from its tip
Footnotes
This study was supported by a Radiological Society of North America Research and Education Fund Seed Grant.
Address reprint requests to Tarik F. Massoud, MD, Section of Neuroradiology, Department of Radiology, University of Cambridge School of Clinical Medicine, Addenbrooke's Hospital, Hills Road, Box 219, Cambridge CB2 2QQ, England.
References
- 1. Mitsubishi Cable Industries, Ltd. Present Status of Development of Medical Instruments and Systems (Review Number 82).. Tokyo, Mitsubishi Cable Industries, Ltd. 1991
- 2.Ramee SR, White CJ. Percutaneous coronary angioscopy. In: Topol EJ, ed. Textbook of Interventional Cardiology. Philadelphia, WB Saunders Company 1994;1122-1135
- 3.Winkelbauer F, Hölzenbein T, Ammann ME, Karnel F, Kretschmer G, Lammer J. Percutaneous angioscopy: improved technique. Cardiovasc Intervent Radiol 1993;16:374-376 [DOI] [PubMed] [Google Scholar]
- 4.Grundfest WS, Litvack F, Sherman T, et al. Delineation of peripheral and coronary detail by intraoperative angioscopy. Ann Surg 1985;202:394-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehigan JT, Olcott C. Video angioscopy as an alternative to intraoperative arteriography. Am J Surg 1986;152:139-145 [DOI] [PubMed] [Google Scholar]
- 6.Miller A, Lipson WE, Isaacsohn JL, Schoen FJ, Lees RS. Intraoperative angioscopy: principles of irrigation and description of a new dedicated irrigation pump. Am Heart J 1989;118:391-399 [DOI] [PubMed] [Google Scholar]