Abstract
BACKGROUND AND PURPOSE: Carbon monoxide intoxication has delayed effects on the cerebral white matter characterized by bilateral, confluent lesions that reflect diffuse demyelination. To increase our understanding of this process, we assessed the diffusion characteristics of these lesions.
METHODS: Five consecutive patients with delayed encephalopathy of CO intoxication were examined with diffusion MR imaging. Diffusion-weighted images (DWIs) were obtained 25–95 days after their exposure to CO and during a relapse of neuropsychiatric symptoms, which occurred after an initial recovery. Imaging was performed at 1.5 T by using a spin-echo echo-planar sequence with diffusion gradients of 0, 500, and 1000 s/mm2. DWIs and apparent diffusion coefficient (ADC) maps were visually evaluated, and mean ADCs were calculated from the periventricular white matter and the centrum semiovale, where confluent hyperintensity was seen on T2-weighted images. Findings were compared with those of normal-looking white matter.
RESULTS: In all five patients, both T2-weighted images and DWIs showed the white matter lesions as bilateral, diffuse, confluent areas of hyperintensity in the periventricular white matter and centrum semiovale. On ADC maps, these lesions were isointense, with focal areas of hypointensity (n = 4) or diffuse hypointensity (n = 1). Mean ADC values of the white matter lesions were significantly lower than those of normal-looking white matter, regardless of their isointensity or hypointensity on ADC maps (P < .05).
CONCLUSION: Bilateral, confluent, white matter lesions in patients with delayed encephalopathy of CO intoxication show decreased diffusivity.
Delayed encephalopathy of carbon monoxide intoxication is clinically characterized by a recurrence of neurologic or psychiatric symptoms. This recurrence is preceded by a temporary asymptomatic period (lucid interval) of variable duration (usually 2–3 weeks) after a recovery from the acute stage of CO intoxication (1, 2). Similar postanoxic delayed encephalopathies may also be seen in cases of respiratory arrest (3), strangling (4), drug overuse, exposure to toxins, anaphylaxis, seizures, or anesthesia (2). The course of the delayed encephalopathy varies with the severity of CO intoxication, ranging from full recovery to a progressive deterioration ending in coma or death. Clinical symptoms and signs include the characteristic symptom triad of mental deterioration, urinary incontinence, and gait disturbance, as well as other neuropsychiatric manifestations (1, 2, 5). The underlying pathologic lesion is thought to be diffuse demyelination of the cerebral white matter (5, 6); however, the pathogenic mechanism remains unexplained. Conventional MR imaging findings of delayed encephalopathy of CO intoxication have been well described (7, 8). T2-weighted images typically show bilateral, symmetric, confluent areas of high signal intensity in the periventricular white matter and centrum semiovale.
Two case reports (9, 10) suggest reduced diffusion in the cerebral white matter in patients with delayed encephalopathy of CO intoxication. The purpose of this study was to investigate these findings on diffusion-weighted images (DWIs) and on apparent diffusion coefficient (ADC) maps and to assess ADC values in the bilateral, confluent lesions in the cerebral white matter in patients with delayed encephalopathy of CO intoxication.
Methods
We retrospectively reviewed six DWIs and ADC maps in five patients (two men, three women; mean age, 63.2 years; age range, 54–71 years) with delayed encephalopathy of acute CO intoxication. In our patients, CO intoxication was diagnosed on the basis of clear evidence of their exposure and a consistent clinical course. Four of the five patients were exposed to CO gas, which leaked from an under-the-floor home heating system, and one patient was exposed exhaust gas in a car. Three patients were found unconscious. They underwent hyperbaric oxygen therapy and then awakened within 24 hours. Two patients had neuropsychiatric symptoms of acute onset, without coma. All patients completely or partially recovered from the initial symptoms within several days to weeks. After apparent lucid intervals of 1–4 weeks, sudden or progressive neuropsychiatric impairments developed again. The patients’ clinical features are summarized in Table 1.
TABLE 1:
Summary of clinical features in five patients with delayed encephalopathy of CO intoxication
| Patient/Age, y/Sex* | Initial Manifestations of Acute CO Intoxication | Lucid Interval, wk | Major Clinical Findings of Delayed Encephalopathy |
|---|---|---|---|
| 1/54/F | Coma with multiple burn injury | 3 | Short-term memory loss, confabulation; much improved at 7-mo follow-up |
| 2/71/M | Sudden decrease of verbal output, free voiding | 1 | Aphasia, gait disturbance; much improved at 5-mo follow-up |
| 3/63/M | Abnormal repetitive behavior, progressive abulia | 3 | Abulia, akinetic mutism; much improved at 8-mo follow-up |
| 4/65/F | Coma, decrease of verbal output and cognitive dysfunction | 4 | Urinary/fecal incontinence, short-stepped gait; persistent clinical symptoms at 6-mo follow-up |
| 5/63/F | Coma, free voiding, and defecation | 4 | Abulia, bradykinesia, free voiding; much improved at 5-mo follow-up |
In all patients except patient 4, the cause of their exposure to CO gas was a gas leakage from an under-the-floor home heating system. Patient 4 was exposed to exhaust gas from a car.
MR images were obtained 25–95 days after CO exposure. In one patient (case 4), images were acquired two times, on days 55 and 82. MR imaging was performed by using a 1.5-T unit (Magnetom Vision Plus; Siemens, Erlangen, Germany). Conventional MR images were obtained with axial T2-weighted fast spin-echo (TR/TE, 4000/98), axial fluid-attenuated inversion recovery (FLAIR) (TR/TE/TI, 9000/110/2200), and sagittal T1-weighted spin-echo (500/20) sequences. DWIs and ADC maps were obtained in the axial plane by using a single-shot spin-echo echo-planar imaging sequence with the following parameters: TR/TE/NEX of 6500/98.9/1, a 128 × 128 matrix, a 24 × 24-cm field of view, and a 5-mm section thickness with a 1–3-mm gap. Diffusion gradients were applied along three orthogonal directions (x, y, and z axes) with three b values (0, 500, and 1000 s/mm2). Iterative nonlinear least-squares fit of a monoexponential decay was used to produce a trace ADC map, which was written on an IDL platform.
Conventional MR images, DWIs, and ADC maps were visually evaluated with the focus on the signal intensity of the white matter lesions. The mean and range of ADC values in the white matter lesions were calculated and compared with those of normal-looking white matter. To obtain the mean values of ADCs in each patient, the ADC value of the white matter lesion was measured in eight regions of interest (ROIs) placed in the lesions in the periventricular white matter and centrum semiovale, as depicted on T2-weighted and FLAIR images. Four ROIs were placed in the areas of low signal intensity, and the other four ROIs were positioned in the areas of isointensity on the ADC map. ADC values of the white matter that appeared normal on T2-weighted and FLAIR images were measured in three ROIs located in the periventricular or subcortical white matter or in the centrum semiovale. The size of each ROI was 2.25 cm2, or 1.125 cm3, containing 64 voxels.
The statistical significance of differences in the mean ADC values of the white matter lesions and the values of normal-looking white matters was calculated with the Mann-Whitney test. A P value of < .05 was considered to indicate a significant difference.
Results
In all five patients, DWIs showed bilateral areas of confluent high signal intensity in the periventricular white matter and centrum semiovale. These areas were similar to those seen on T2-weighted and FLAIR images (Figs 1–3). The hyperintensity varied from slight to severe. In three patients, the finding was more intense and prominent in the frontal lobes than in other regions (Fig 1). In four patients, images showed diffuse, symmetric distribution of the lesions throughout the cerebral white matter. In the remaining patient (case 2), the white matter lesions appeared asymmetric (Fig 2). The abnormality had slight hypointensity or isointensity.
Fig 1.
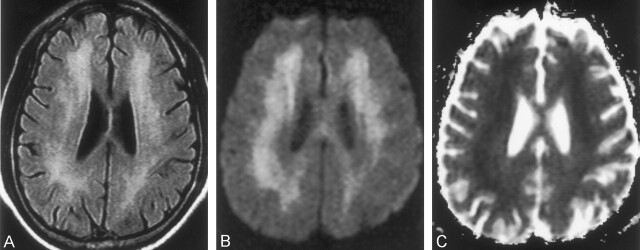
Case 1. MR images obtained in a 54-year-old woman on day 25 after CO exposure.
A and B, T2-weighted images (TR/TE/NEX, 4000/98/1) show bilateral, symmetric, confluent areas of high signal intensity in both the centrum semiovale (A) and periventricular white matter (B). The high intensity appears more prominent in the frontal lobes than elsewhere.
C and D, ADC maps (calculated from DWIs [6500/98/1, b = 0 and 1000 s/mm2]) obtained at the same levels as in A and B, respectively, show focal areas of low signal intensity (decreased diffusion) in the bilateral frontal lobes. Most of the remaining centrum semiovale and periventricular white matter appears isointense. DWIs at the same levels showed confluent high signal intensity in the centrum semiovale and periventricular white matter. The appearance was similar to that on the T2-weighted images (not shown) and mainly resulted from a T2 shine-through effect.
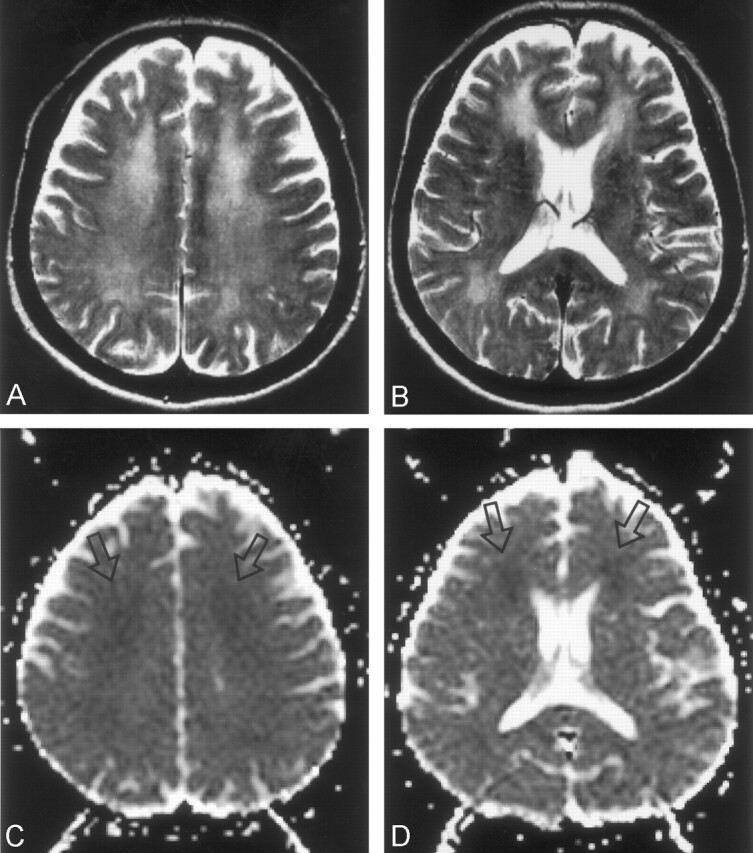
Fig 3.
Case 4. MR images obtained in a 65-year-old woman on day 55 after CO exposure.
A, FLAIR image (TR/TE/TI/NEX, 9000/110/2200/1) shows diffuse high-intensity lesions in the periventricular white matter.
B, DWI (TR/TE/NEX, 6500/98/1; b = 1000 s/mm2) shows confluent high signal intensity in the same periventricular white matter.
C, ADC map shows diffuse low signal intensity in the periventricular white matter.
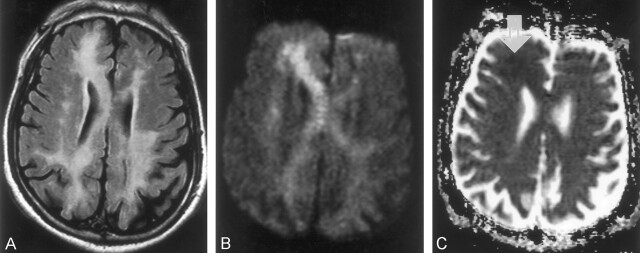
Fig 2.
Case 2. MR images obtained in a 71- year-old man on day 41 after CO exposure.
A, FLAIR image (TR/TE/TI/NEX, 9000/110/2200/1) shows asymmetric distribution of the high-intensity lesions in the periventricular white matter and corpus callosum.
B, DWI (TR/TE/NEX, 6500/98/1; b = 1000 s/mm2) shows similar high signal intensity in the periventricular white matter and corpus callosum. The high intensity is more prominent in the right frontal lobe and on the right side of the genu of the corpus callosum than elsewhere.
C, ADC map shows a focal area of subtle low signal intensity in the right frontal lobe (arrow). Other white matter lesions appear isointense.
In four patients, most of the white matter lesions appeared isointense relative to the normal-looking white matter on ADC maps, as seen on T2-weighted and FLAIR images. Focal areas of low signal intensity were located mainly in the frontal lobes (Figs 1, 2). In the remaining patient (case 4), who had persistent neuropsychiatric impairment at 6-month clinical follow-up, the white matter lesions had diffuse low signal intensity on the ADC maps (Fig 3). The 1-month follow-up MR image of these lesions showed no substantial interval changes in the ADC.
In all five patients, mean ADC values of the white matter lesions were significantly lower than those of normal-looking white matter, regardless of their isointensity or hypointensity on ADC maps (P < .05). Mean ADC values of the hypointense or isointense white matter lesions, as seen on ADC maps, were 0.46–0.68 10−3 mm2/s or 0.70–0.85 10−3 mm2/s, respectively, whereas the mean ADC value of normal-looking white matter was 0.86–0.94 10−3 mm2/s, P < .05). The mean ADC value was the lowest in the patient with persistent neuropsychiatric impairment on follow-up (case 4), whereas it was the highest in the patient with relatively mild neuropsychiatric symptoms (case 5).
The findings on conventional MR images, DWIs, and ADC maps and the ADC values in the white matter lesions are summarized in Table 2.
TABLE 2:
Summary of findings on conventional MR images, DWIs, and ADC maps, with ADC values in five patients with delayed encephalopathy of CO intoxication
| Patient | Interval to Imaging, d* | Findings on T2-Weighted and FLAIR Images | Findings on DWIs | Findings on ADC Maps | Mean ADC Values, ×10−3 mn |
|---|---|---|---|---|---|
| 1 | 25 | Diffuse high intensity in the PVWM and centrum semiovale, more prominent in both frontal lobes; high intensity in both globi pallidus | Diffuse high intensity in the PVWM and centrum semiovale, more prominent in both frontal lobes | Focal areas of low intensity in both frontal lobes | 0.61 (0.53–0.70)/0.89 |
| 2 | 41 | Asymmetric high intensity in the PVWM, centrum semiovale, and corpus callosum; more prominent on R side | Asymmetric high intensity in the PVWM, centrum semiovale, and corpus callosum; more prominent on the R side | Focal areas of low intensity in R frontal lobe | 0.65 (0.53–0.75)/0.91 |
| 3 | 56 | Diffuse high intensity in the PVWM and centrum semiovale | Diffuse high intensity in the PVWM and centrum semiovale | Focal areas of low intensity in both frontal lobes | 0.64 (0.55–0.73)/0.93 |
| 4 | 55 | Diffuse high intensity in the PVWM and centrum semiovale | Diffuse high intensity in the PVWM and centrum semiovale | Diffuse low intensity in the PVWM | 0.56 (0.46–0.71)/0.86 |
| 4‡ | 82 | No change | No change | No change | 0.53 (0.41–0.71)/0.91 |
| 5 | 95 | Diffuse high intensity in the PVWM and centrum semiovale, more prominent in both frontal lobes; high intensity in both globi pallidus | Diffuse high intensity in the PVWM and centrum semiovale | Focal areas of low intensity in both frontal lobes | 0.75 (0.68–0.85)/0.94 |
Note.—PVWM indicates periventricular white matter.
Interval between CO Exposure and MR Imaging.
†Data are the value in the WM lesions (range)/value in normal-looking WM on T2-weighted images.
Follow-up MR examination.
Discussion
The principal neuropathologic feature of delayed encephalopathy of CO intoxication is diffuse white matter abnormalities. Three types of white matter abnormalities have been described (5, 6): multifocal necrotic lesions predominantly within the centrum semiovale and commissures, extensive necrotic zones in the periventricular white matter with extension across the corpus callosum and commissures, and patchy or extensive demyelination of the periventricular and deep central white matter. Selective white matter demyelination is thought to be more closely associated with delayed postanoxic encephalopathy, whereas white matter necrosis is more closely associated with a monophasic, nonrelapsing illness. However, the clinical and pathologic findings may overlap. The pathogenesis of the delayed encephalopathy of CO intoxication remains uncertain.
The results of the present study indicate that the white matter lesions have decreased diffusivity, although in four patients, most white matter lesions appeared isointense on the ADC map. In those patients, the high signal intensity on DWI is thought to be mainly due to a T2 shine-through effect. Our results suggest that, in delayed encephalopathy of CO intoxication, the restricted diffusion of the white matter lesion develops late after the patient’s exposure to CO and persists longer than usual 2–3 weeks after an acute ischemic infarct. In acute stroke, the ADC value is most reduced at 8–32 hours, it remains markedly reduced for 3–5 days, and it returns to baseline at 1–4 weeks (11). Acute cell necrosis with cytotoxic edema, as seen in acute ischemic infarction, does not seem to occur. However, tissue injury caused by slowly progressive cytotoxic edema related to the direct toxic effect of CO gas or some other unknown mechanism results in reduced diffusion in the delayed encephalopathy of CO intoxication. The latency of the clinical symptoms, the delayed appearance of MR imaging findings (including decreased diffusivity), and the pathologic findings might reflect late-developing biochemical changes that becomes evident when some metabolites are accumulated.
Murata et al (9) described serial diffusion imaging findings in a case of delayed encephalopathy of CO poisoning. ADC values in the frontal white matter appeared to be within the normal range on day 23 after CO exposure, but they progressively decreased until day 38 after exposure, then slowly increased. The values were higher values at day 118 after exposure than they were immediately after the appearance of sequelae. In one of two patients with CO intoxication reported by Teksam et al (10), follow-up MR imaging was performed 2 months after their initial admission, when the neurologic symptoms of delayed encephalopathy developed. The images showed additional aggravation of the lesions in bilateral periventricular white matter and centrum semiovale, with new development of subtle restriction of water diffusion in the cerebral white matter. This last finding completely resolved on another follow-up image obtained 10 months after the initial admission. The MR imaging findings in our cases were similar to those of the previous reports (10, 11). One patient (case 4) (Fig 3) of the present series did not have clinical improvement at 6-month follow-up, and the mean ADC values of the white matter lesions remained decreased on days 55 and 82.
Singhal et al (12) reported diffusion MR imaging findings in three patients of anoxic encephalopathy. One had acute CO intoxication, and diffusion MR imaging performed 3 days after the patient’s exposure to CO gas showed decreased ADC values in the bilateral globi pallidus, the hippocampus, and temporal and occipital cortices. No abnormality was observed in the periventricular white matter or centrum semiovale, unlike the white matter lesions noted in delayed encephalopathy of CO intoxication. However, Chalela et al (13) described symmetric areas of restricted diffusion in the periventricular white matter and centrum semiovale, which were observed in seven patients with anoxic-ischemic encephalopathy. These patients included one with acute CO intoxication who underwent brain MR imaging within 7 days of the insult. The authors speculated that, in their patients, the low ADC values related to acute myelinopathy likely represented early cytotoxic edema secondary to cellular energy failure. Unfortunately, further follow-up imaging was not performed in their study.
The duration and degree of the decreased diffusivity in the white matter may vary, and it may be correlated with the clinical outcome, depending on the severity of the CO intoxication. In the present study, patient 4 (Fig 3) had the lowest ADC value and an area of low signal intensity on the ADC map that was wider than that of the other patients. This patient also had a poorer clinical course and a smaller area of focal low signal intensity on the ADC map. This observation suggests that the extent and degree of low ADC values in the white matter are correlated with the clinical course, and such findings may possibly enable us to predict the prognosis. Further systematic studies of diffusion imaging, including diffusion tensor imaging, with larger number of patients are needed to assess the prognosis and to clarify the pathogenesis of delayed encephalopathy of CO intoxication.
Conclusion
In all five patients with delayed encephalopathy of CO intoxication, DWIs showed diffuse, confluent areas of high signal intensity in the periventricular white matter and centrum semiovale. These areas were similar to those seen on T2-weighted and FLAIR images.
On ADC maps, the white matter lesions appeared as either diffuse isointensity with focal hypointensity (n = 4) or diffuse hypointensity (n = 1). Mean ADC values of the white matter lesions were significantly lower than those of normal-looking white matter in all five patients, regardless of the isointensity or hypointensity on ADC maps. We speculate that late-developing cytotoxic edema or some other mechanism related to the accumulation of unknown toxic biochemical products results in reduced diffusion. Further studies of diffusion imaging with larger numbers of patients are needed to correlate the extent and degree of low ADC values in the white matter with the clinical outcomes and prognosis.
Footnotes
This study was supported in part by the 2002 BK21 Project for Medicine, Dentistry, and Pharmacy.
References
- 1.Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol 1983;40:433–435 [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg MD. Delayed neurological deterioration following hypoxia. Adv Neurol 1979;26:21–44 [PubMed] [Google Scholar]
- 3.Roychowdhury S, Maljian JA, Galetta SL, Grossman RI. Postanoxic encephalopathy: diffusion MR findings. J Comput Assist Tomogr 1998;22:992–994 [DOI] [PubMed] [Google Scholar]
- 4.Dooling EC, Richardson P Jr. Delayed encephalopathy after strangling. Arch Neurol 1976;33:196–199 [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg MD, Myers RE, McDonagh BF. Experimental carbon monoxide encephalopathy in the primate, II: clinical aspects, neuropathology, and physiologic correlation. Arch Neurol 1974;30:209–216 [DOI] [PubMed] [Google Scholar]
- 6.Lapresle J, Fardeau M. The central nervous system and carbon monoxide poisoning, II: anatomical study of brain lesions following intoxication with carbon monoxide. Prog Brain Res 1967;24:31–74 [DOI] [PubMed] [Google Scholar]
- 7.Chang KH, Han MH, Kim HS, Wie BA, Han MC. Delayed encephalopathy after acute carbon monoxide intoxication: MR imaging features and distribution of cerebral white matter lesions. Radiology 1992;184:117–122 [DOI] [PubMed] [Google Scholar]
- 8.Horowitz AL, Kaplan R, Sarpel G. Carbon monoxide toxicity: MR imaging in the brain. Radiology 1987;162:787–788 [DOI] [PubMed] [Google Scholar]
- 9.Murata T, Kimura H, Kado H, et al. Neuronal damage in the interval form of CO poisoning determined by serial diffusion weighted magnetic resonance imaging plus 1H-magnetic resonance spectroscopy. J Neurol Neurosurg Psychiatry 2001;71:250–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teksam M, Casey SO, Michel E, Liu H, Truwit CL. Diffusion-weighted MR imaging findings in carbon monoxide poisoning. Neuroradiology 2002;44:109–113 [DOI] [PubMed] [Google Scholar]
- 11.Schaefer PW, Grant E, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology 2000;217:331–345 [DOI] [PubMed] [Google Scholar]
- 12.Singhal A, Topcuoglu MA, Koroshetz WJ. Diffusion MRI in three types of anoxic encephalopathy. J Neurol Sciences 2002;196:37–40 [DOI] [PubMed] [Google Scholar]
- 13.Chalela JA, Wolf RL, Maldjian JA, Kasner SE. MRI identification of early white matter injury in anoxic-ischemic encephalopathy. Neurology 2001;56:481–485 [DOI] [PubMed] [Google Scholar]