Abstract
BACKGROUND AND PURPOSE: Our newly developed biocompatible embolic materials, hydroxyapatite ceramic microparticles, have good visibility during injection control and were shown to be capable of producing effective occlusion of the distal arteriocapillary bed in an experimental animal study. The purpose of this present study was to evaluate hydroxyapatite ceramic microparticles for use in human meningioma embolization.
METHODS: Thirteen patients with meningiomas underwent preoperative superselective embolization with the use of hydroxyapatite microparticles. Radiologic and histopathologic studies of the surgical specimens were performed.
RESULTS: During embolization, no microcatheter clogging was observed and angiographic devascularization was consistently obtained without unexpected proximal occlusions. Histopathologic findings showed that there was mild inflammatory response in the thrombosed lumen.
CONCLUSION: Hydroxyapatite microparticles are excellent embolic materials for the treatment of human meningioma. They have excellent biocompatibility and good injection control, which produces occlusion of the distal arteriocapillary bed.
Preoperative embolization of meningiomas was initially reported by Manelfe et al in 1973 (1) and has come to be a widely recognized preoperative procedure aimed at devascularization and obliteration of the arteriocapillary beds (2–5). In our preliminary study, we evaluated the characteristics and embolic properties of hydroxyapatite microparticles, both angiographically and histopathologically, in experimental animal embolization. Hydroxyapatite microparticles proved to be an effective and safe embolic material. The present clinical study was undertaken to evaluate these hydroxyapatite microparticles, both angiographically and histopathologically, in patients with meningiomas.
Methods
All the procedures were performed after approval of the Committee on Medical Ethics of Toyama Medical and Pharmaceutical University and after informed consent was obtained from the patients and/or their next of kin. Thirteen patients (six female, seven male; mean age, 56.5 years; age range, 35–73 years) with meningiomas at various locations (convexity, n = 6; sphenoid ridge, n = 3; parasagittal, n = 3; falx, n = 1) were enrolled in our study. Pre-embolization MR imaging and superselective cerebral angiography were performed for all patients. Hydroxyapatite microparticles (produced at 800°C), ranging between 100 and 250 μm in diameter, were sterilized by ethylene oxide gas during 24 hr before use. The suspension of hydroxyapatite was made in concentration, with 10 mg for 10 mL of 80% contrast media before injection. Microcatheters with transparent hubs were selected so that injection control of embolic materials could be visually monitored to prevent clogging of microcatheters. The transfemoral approach was used in all patients along with heparinization and local anesthesia. Diagnostic superselective angiography was performed immediately before embolization. Embolization was performed only in external carotid system feeders for preoperative purposes, and it was not performed in internal carotid artery feeders. After superselective catheterization of external carotid artery feeders, provocative testing with lidocaine and thiopental sodium was performed to check whether these arteries supplied any cranial nerves anastomosed to the ophthalmic arteries or cerebral arteries. Embolization was performed manually under fluoroscopic monitoring only for feeders that had negative results of provocative testing. Injection was discontinued when stagnation of flow was encountered.
After embolization, devascularization effect in each feeder was classified into two groups: group A (95%–100%) and group B (<95%). Patency of cannulated feeding arteries was checked angiographically immediately after embolization to detect proximal feeding arterial occlusion due to particle aggregation. Evaluation was performed by two neurosurgeons who were blinded to this study. In several feeders with positive results of the provocative testing, we used microcoils to obtain proximal occlusion; these vessels were not included in this study. The interval between embolization and surgery varied from 2 to 22 days (mean, 7.3 days). All the patients underwent surgery while under general anesthesia. After total or subtotal tumor removal, the sections of surgical specimens were examined with hematoxylin and eosin staining after incomplete decalcification.
Results
Embolization with hydroxyapatite microparticles was successfully performed in all 13 cases, for a total of 27 feeding arteries in meningiomas in various locations with no resulting general, focal, or neurologic complications. We experienced no microcatheter clogging during the embolization procedures. Microparticle injection was consistently easy. Control of injection was excellent because of the good visibility of the embolic agent, especially at the level of microcatheter hub. Tumor vascularity during the arterial to capillary phase was consistently noted to gradually decrease and finally disappear in all cases, so that tumor stain was not present during the venous phase. The devascularization grade of the vessels treated was A in all cases (Table). Unexpected feeder occlusion did not occur in any cases, and all the feeders were patent immediately after embolization, consistent with effective delivery of particles to the tumor arteriocapillary beds.
Summary of 13 patients with meningioma embolized with hydroxyapatite microparticles
| No. Patient | Age (yr)/Sex | Location | Feeders Embolized with HAP (Embolization Grade) |
|---|---|---|---|
| 1 | 35/F | Vault | MMA-p (A) |
| 2 | 55/M | Vault | MMA-p (A) |
| 3 | 59/F | Vault | MMA-a (A) |
| 4 | 63/M | Vault | MMA-a (A), MMA-p (A), AMA (A) |
| 5 | 64/M | Vault | MMA-a (A), MMA-p (A) |
| 6 | 73/M | Vault | MMA (A), AMA (A) |
| 7 | 41/F | Sphenoidal ridge | MMA-a (A) |
| 8 | 44/F | Sphenoidal ridge | MMA-a (A), MMA-p (A) |
| 9 | 53/F | Sphenoidal ridge | MMA-a (A), AMA (A) |
| 10 | 65/M | Parasagittal | MMA (A) |
| 11 | 66/M | Parasagittal | MMA-a (A), MMA-p (A) |
| 12 | 66/M | Parasagittal | MMA-p (A) |
| 13 | 53/F | Falx | MMA-a (A) |
Note.—HAP indicates hydroxyapatite; F, female; M, male; MMA-p, posterior branch of the middle meningeal artery; MMA-a, anterior branch of the middle meningcal artery; AMA, accessory meningeal artery.
All 13 patients underwent total or subtotal tumor surgical removal. Histologic evaluation of incompletely decalcified specimens disclosed mild inflammatory response and lymphocytic infiltration in the perivascular spaces (Fig 1). No hemorrhage or angionecrosis was found in any of the specimens.
Fig 1.
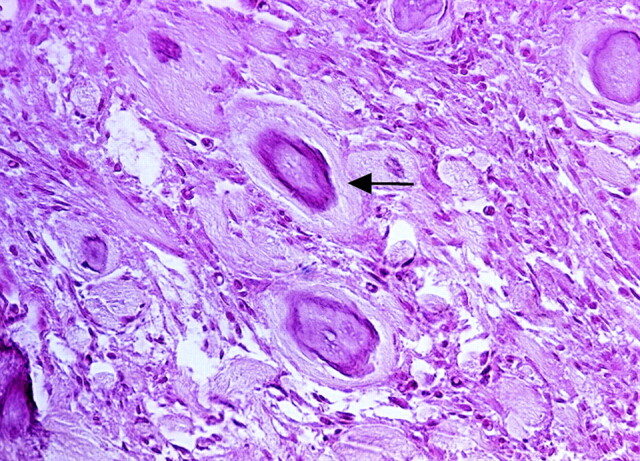
Hydroxyapatite microparticles (arrow) are seen in small arteries in meningioma with mild inflammatory response and lymphocytic infiltration in the perivascular spaces (hematoxylin and eosin; original magnification, ×100).
Discussion
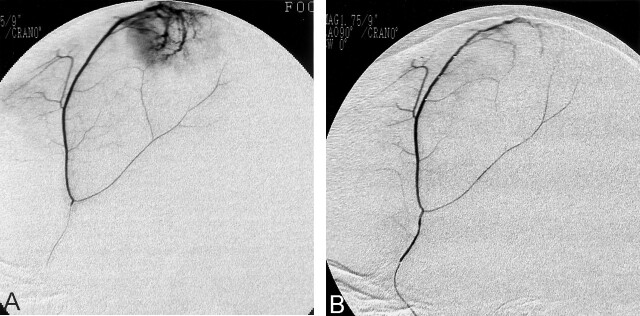
Radiologic evidence of diffuse embolization consists of two findings: the first is complete devascularization of the tumor on angiograms, and the second is complete patency of the feeders immediately after devascularization of the tumor, consistent with obliteration of the distal arteriocapillary bed without unexpected proximal occlusion of feeders. From this standpoint, our material showed satisfactory results (Fig 2A and B). Recent advancements in microcatheter and microwire technology and digital subtraction angiography enabled us to perform tumor embolization much easier and safer than before. Appropriate selection of the cases, provocative testing before embolization, and careful use of embolic agents have also helped to reduce complications. However, several reports of recanalization after embolization have been published (4, 6), as have reports of complications of unclear cause, some of which may be related to the characteristics of the embolic materials themselves (6–8), such as intratumoral or peritumoral hemorrhage around the occluded vessels (9–11), edema around the occluded vessels (10), and AVF.
Fig 2.
Superselective angiograms of the middle meningeal artery feeding a frontal parasagittal meningioma.
A, Before embolization.
B, Immediately after embolization with hydroxyapatite microparticles. The meningioma is angiographically devascularized.
An advanced inflammatory response is considered to cause arterial necrosis and hemorrhage. Subcutaneous tissue implantation is said to be more sensitive for detecting inflammation reaction to embolic materials than implantation in other organs, especially the brain (12–14). Misiek et al (15) showed a very mild inflammatory response with hydroxyapatite particles in subcutaneous tissue, but polyvinyl alcohol particles caused extensive fibrosis in subcutaneous tissue (16). In this regard, hydroxyapatite produces milder inflammation than does polyvinyl alcohol particles. On the other hand, early recanalization sometimes results from much less or almost no inflammatory response.
We experienced no hemorrhage or recanalization during a follow-up period of ≤24 weeks after embolization with our particles in our preliminary animal study. In the histopathologic section of resected meningiomas, hemorrhage and angionecrosis were not shown and expected mild inflammatory signs were shown, as were those of our animal experimental study. The ideal benign inflammation reaction may be obtained with hydroxyapatite particles.
More recently, it has been suggested that hydroxyapatite ceramics in a porous configuration be used as a drug or antibody release system, which is another unique characteristic of hydroxyapatite (7). These microparticles may even have a potential use as chemotherapeutic embolic material in the future.
Conclusion
In conclusion, hydroxyapatite ceramic microparticles constitute an excellent biocompatible embolic material with good visibility during injection control. They are capable of producing effective occlusion of distal arteriocapillary beds, with a resulting mild benign inflammatory response and preservation of the arterial feeders.
References
- 1.Manelfe C, Guiraud B, David J. Embolization by catheterization of intracranial meningiomas [in French]. Rev Neurol Paris 1973;128:339–351 [PubMed] [Google Scholar]
- 2.Richter HP, Schachenmayr W. Preoperative embolization of intracranial meningiomas. Neurosurgery 1983;13:261–268 [DOI] [PubMed] [Google Scholar]
- 3.Castaneda-Zuniga WR, Sanchez R, Amplatz K. Experimental observations on short and long-term effects of arterial occlusion with Ivalon. Radiology 1978;126:783–785 [DOI] [PubMed] [Google Scholar]
- 4.Teasdale E, Patterson J, McLellan D, Mcpherson P. Subselective preoperative embolization for meningiomas: a radiological and pathological assessment. J Neurosurg 1984;60:506–511 [DOI] [PubMed] [Google Scholar]
- 5.Manelfe C, Lasjaunias P, Ruscalleda J. Preoperative embolization of intracranial meningiomas. AJNR Am J Neuroradiol 1986;7:963–972 [PMC free article] [PubMed] [Google Scholar]
- 6.Kurata A, Saegusa H, Ohmomo T, et al. Preoperative embolization for meningiomas using PVA particles [in Japanese]. No Shinkei Geka 1992;20:367–373 [PubMed] [Google Scholar]
- 7.Netz DJ, Sepulveda P, Pandolfelli VC, et al. Potential use of gelcasting hydroxyapatite porous ceramic as an implantable drug delivery system. Int J Pharm 2001;213:117–125 [DOI] [PubMed] [Google Scholar]
- 8.Wakhloo AK, Juengling FD, Van Velthoven V, et al. Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: assessment of two embolization techniques. AJNR Am J Neuroradiol 1993;14:571–582 [PMC free article] [PubMed] [Google Scholar]
- 9.Turjman F, Massoud TF, Vinters HV, et al. Collagen microbeads: experimental evaluation of an embolic agent in a rete mirabile of the swine. Am J Neuroradiol 1995;16:1031–1036 [PMC free article] [PubMed] [Google Scholar]
- 10.Dion JE, Rankin RN, Viñuela F, Fox AJ, Wallace AC, Mervart M. Dextran microsphere embolization: experimental and clinical experience with radiologic-pathologic correlation: work in progress. Radiology 1986;160:717–721 [DOI] [PubMed] [Google Scholar]
- 11.Purdy PD, Samson D, Batjer HH, Risser RC. Preoperative embolization of cerebral arteriovenous malformations with polyvinyl alcohol particles: experience in 51 adults. AJNR Am J Neuroradiol 1990;11:501–510 [PMC free article] [PubMed] [Google Scholar]
- 12.Sadato A, Taki W, Ikada Y, et al. Experimental study and clinical use of poly(vinyl acetate) emulsion as liquid embolisation material. Neuroradiology 1994;36:634–641 [DOI] [PubMed] [Google Scholar]
- 13.Schweitzer JS, Chang BS, Madsen P, et al. The pathology of arteriovenous malformations of the brain treated by embolotherapy: II. results of embolization with multiple agents. Neuroradiology 1993;35:468–474 [DOI] [PubMed] [Google Scholar]
- 14.Lanman TH, Martin NA, Vinters HV. The pathology of encephalic arteriovenous malformations treated by prior embolotherapy. Neuroradiology 1988;30:1–10 [DOI] [PubMed] [Google Scholar]
- 15.Misiek DJ, Kent JN, Carr RF. Soft tissue responses to hydroxyapatite particles of different shapes. J Oral Maxillofac Surg 1984;42:150–160 [DOI] [PubMed] [Google Scholar]
- 16.Chen RW, Musser AW, Postlethwait RW. Alterations of and tissue reaction to polyvinyl alcohol sponge implants. Surgery 1969;66:899–906 [PubMed] [Google Scholar]