Abstract
BACKGROUND AND PURPOSE: Ipsilateral loss of anterior temporal gray-white matter definition, due mainly to white matter signal intensity abnormality, is frequently seen on MR images of patients with hippocampal sclerosis. Our aim was to determine the prevalence and clinical correlations of these anterior temporal changes in pediatric cases of hippocampal sclerosis and to determine whether cumulative damage from seizures is important for their development.
METHODS: We reviewed the MR images and clinical details of 54 children (age range, 1.5–19 years) with typical hippocampal sclerosis. Specific imaging features noted included hippocampal sclerosis, anterior temporal changes, anterior temporal atrophy, and extra-hippocampal abnormality.
RESULTS: Thirty-one (57%) of 54 children with hippocampal sclerosis had associated ipsilateral anterior temporal changes. Ipsilateral anterior temporal atrophy was associated with anterior temporal changes (P < .03). Children whose images showed anterior temporal changes were younger at onset of epilepsy (P < .01) and younger at antecedent cerebral insult (P < .03) than those with normal anterior temporal lobes. Most (84%) children whose images showed anterior temporal changes had experienced the onset of epilepsy or antecedent cerebral insult before the age of 2 years (P < .0009). Eighty-one percent of children with anterior temporal changes shown on their images experienced seizures at the time of antecedent insult.
CONCLUSION: Ipsilateral anterior temporal changes identical to those observed in adult cases are seen on the MR images of young children with hippocampal sclerosis, with a similar prevalence, and are associated with either epilepsy onset or seizure-related cerebral insult before the age of 2 years. We suggest that the loss of gray-white matter definition may represent a persistent immature appearance, including an abnormality of myelin or myelination, possibly a result of seizures occurring during maturation of the temporal pole.
Ipsilateral anterior temporal changes are described as part of the spectrum of MR imaging features found in adult cases of hippocampal sclerosis and in cases of intractable temporal lobe epilepsy per se (1–3). This abnormality typically consists of loss of gray-white matter definition, with abnormal signal intensity predominantly in white matter, best seen on T2-weighted images (Figs 1–4), with signal intensity abnormality also visible on T1-weighted images in some cases (Fig 4). The prevalence in adult cases ranges between 32% and 66% (3, 4). Ipsilateral temporal atrophy is associated with both anterior temporal changes (2) and hippocampal sclerosis (1, 5). The clinical correlations and underlying histopathology of these anterior temporal changes have not been conclusively established in adults (1–4, 6–9). Also, it is not known whether the imaging abnormality reflects damage from chronic epilepsy or early injury or whether anterior temporal changes are present before the onset of epilepsy. These anterior temporal changes have been labeled dual pathology by some authors (6–9). We prefer to use a more descriptive label because uncertainty still exists regarding the underlying histology and pathogenesis. The same appearance has also been termed temporo-polar MRI abnormalities (10). However, because these appearances often extend beyond the temporal pole, we prefer the term anterior temporal changes.
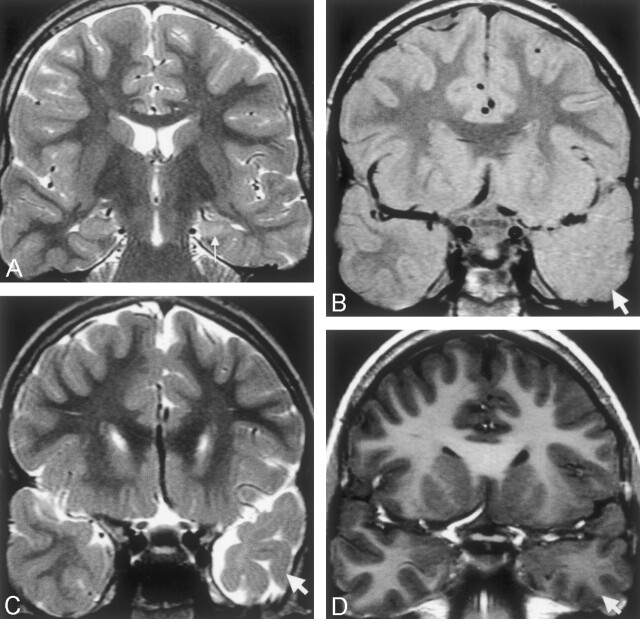
Fig 1.
Images of a 10-year-old male patient who had meningitis at age 6 months and then onset of temporal lobe epilepsy at age 3 years.
A, Coronal view fast spin-echo image (2975/98) shows typical left hippocampal sclerosis, with loss of volume and increased signal intensity in the left hippocampus (arrow).
B and C, Coronal view proton attenuation-weighted images (2975/17) show lack of anterior temporal gray-white matter definition (arrows) and atrophy compared with the normal right side.
D, Coronal view fast spoiled gradient-recalled volume acquisition T1-weighted image shows subtle decreased signal intensity within the anterior temporal white matter compared with the left side, with no cortical thickening.
Fig 4.
Images of a 22-month-old male patient with severe left temporal lobe epilepsy that was recognized at age 9 months after bacterial meningitis at age 6 months.
A and B, Coronal view T2-weighted fast spin-echo images (2975/98) show an immature myelin pattern in the right temporal pole and frontal poles. The left anterior temporal lobe white matter shows abnormal increased signal intensity (arrows) compared with the normal right side with subtle ipsilateral atrophy.
C, Coronal view heavily T1-weighted inversion recovery image (8000/68; inversion time, 400 ms) shows left hippocampal sclerosis (arrowhead) and abnormal decreased signal intensity within temporal white matter (asterisk). Abnormal decreased signal intensity within the temporal, insular, and inferior frontal cortex also can be seen, compared with the right side (small arrows), with no cortical thickening.
We have observed anterior temporal changes in pediatric cases of epilepsy and hippocampal sclerosis, with appearances identical to those found in adult cases. With the current study, our aim was to examine the prevalence and clinical correlations of anterior temporal changes in pediatric cases of hippocampal sclerosis. In addition, we aimed to determine whether these changes occur early during the course of epilepsy or whether they are seen less often in pediatric cases than in adult cases, which might imply that this is an acquired abnormality. We postulate that if anterior temporal changes occur as a result of damage induced by repeated seizures occurring over a long period, then prevalence is likely to be lower in children.
Methods
We searched the radiology database at our institution, a tertiary pediatric hospital, for cases of possible hippocampal sclerosis revealed by MR imaging performed between 1991 and 2001. We identified 64 children with radiologic reports suggesting hippocampal sclerosis. We identified 52 control images of children of similar ages by searching the database for patients who had undergone MR imaging examinations for minor non-seizure symptoms, such as headaches, and who had a normal radiologic report. All patients and control participants had undergone imaging on a 1.5-T system (GE, Milwaukee, WI), with pulse sequences that included tilted coronal view T2-weighted and proton attenuation-weighted (2975/17–98/3 [TR/TE/number of excitations]) fast spin-echo images angled perpendicular to the long axis of the hippocampus, axial view T2-weighted (4600/102/3) fast spin-echo images, and a fast spoiled gradient-recalled volume acquisition (17.7/3.4 [TR/minimum TE]; inversion time, 300 ms) with tilted coronal, axial, and sagittal view reformatted images.
Patient and control images were mixed together, and three experienced radiologists who were blinded to the clinical information and presence of hippocampal sclerosis assessed the presence of anterior temporal changes. The purpose of the control images was to ensure that the detected anterior temporal changes did represent abnormal appearance rather than artifact. Initially, coronal view T2-weighted and proton attenuation-weighted images showing only the anterior temporal lobes were assessed for anterior temporal changes, with the hippocampi and the rest of the brain obscured. Images were first reviewed independently, and in the rare cases of disagreement, the images were reviewed together, resulting in a consensus report. All available images were then assessed for the presence of hippocampal sclerosis and other pathologic abnormalities. MR imaging assessment was limited to a qualitative assessment; volumetric analysis was not performed. When follow-up MR imaging examinations were available, they were reviewed to assess whether anterior temporal changes developed over a time interval or, if anterior temporal changes were present on the initial images, whether they had increased in severity after an interval.
Anterior temporal changes were defined as loss of gray-white matter definition on T2-weighted and proton attenuation-weighted images with abnormal increased white matter signal intensity, with or without visible atrophy, as previously described (1–3, 10). Hippocampal sclerosis was diagnosed according to previously recognized criteria: loss of hippocampal volume, increased hippocampal signal intensity on T2-weighted images, decreased hippocampal signal intensity on T1-weighted images, and loss of internal hippocampal architecture (11, 12).
After the MR imaging assessment, we reviewed the clinical histories of patients with definite hippocampal sclerosis. Clinical details noted included age, sex, age at onset of epilepsy, type of and age at occurrence of any antecedent cerebral insult (including febrile convulsions), time from insult to epilepsy onset, and duration of epilepsy before MR imaging. Seizure frequency and overall number of seizures were estimated. Seizure frequency during the 6 months before the MR imaging examination was also estimated. In addition, we noted any family history of epilepsy and whether surgery had been performed. The pathologic reports of the resected temporal lobe specimens were noted in surgically treated cases.
Statistical analyses included χ2 tests for all contingency data and Student’s t tests for comparison of the groups as stated. P < 0.05 was considered statistically significant. This study was performed in accordance with the principles governing human research as determined by the Ethics and Human Research Committee at our institution.
Results
MR Imaging
Review of the 64 patients with previously reported hippocampal sclerosis revealed that only 55 patients had definite hippocampal sclerosis. The nine patients with normal hippocampi and no anterior temporal changes on blinded review were not evaluated further. One additional patient with hippocampal sclerosis and hydrocephalus was not included in the following analysis because his anterior temporal lobes could not be readily classified because of markedly dilated temporal horns. Our hippocampal sclerosis study group therefore consisted of 54 patients (31 female and 23 male patients; age range, 1.5–19 years; mean age, 11.3 years): 51 with unilateral hippocampal sclerosis (26 right, 25 left) and three with bilateral hippocampal sclerosis. The control images of 52 children of similar ages (age range, 3.7–17.5 years; mean age, 11.5 years) showed that none had hippocampal sclerosis or other significant abnormalities; incidental findings included two choroidal fissure cysts, one small pineal cyst, and one mild plagiocephaly.
Thirty-one (57%) of 54 children with hippocampal sclerosis had anterior temporal changes, and 23 (43%) had no anterior temporal changes. Anterior temporal changes occurred only on the side of the hippocampal sclerosis in 29 cases of unilateral hippocampal sclerosis. In the two patients with anterior temporal changes and bilateral hippocampal sclerosis, one had bilateral anterior temporal changes and the other had anterior temporal changes only on the side of the most severe hippocampal atrophy. The side of hippocampal sclerosis made no difference regarding the presence of anterior temporal changes (P < .86, χ2 test). Ipsilateral anterior temporal atrophy was present in 12 patients with anterior temporal changes and in three without anterior temporal changes (P < .03, χ2 test). Extra-hippocampal pathologic abnormality was found in eight patients with anterior temporal changes and in 10 with no anterior temporal changes (previous hypoxic ischemic encephalopathy or periventricular leukomalacia in five, focal or diffuse gliosis in seven, microcephaly in one, ganglioglioma in one, previous cerebral infarcts in three, and multiple cavernomas in one) (P < .17, χ2 test). None of the control images showed anterior temporal changes.
The MR imaging features of anterior temporal changes in pediatric cases were the same as those previously described in adult cases (1–3, 10). Typically, gray matter and white matter were of similar signal intensity on T2- and proton attenuation-weighted images (Figs 1 and 2). Two patients with anterior temporal changes underwent imaging before development of a mature anterior temporal myelin pattern. Both underwent imaging just more than a year after epilepsy onset and showed considerably increased signal intensity within the temporal pole white matter on T2-weighted images (Figs 3 and 4). One of these patients underwent follow-up imaging at age 5 years; by that age, similar signal intensity was observed between gray and white matter, with reduced white matter hyperintensity, similar to the pattern of anterior temporal signal intensity changes observed in adult cases (Fig 3). On T1-weighted fast spoiled gradient-recalled volume acquisition images, subtle decreased white matter signal intensity (Figs 1 and 4) was observed in 14 cases but flow artifact obscured detail in many cases. No evidence of cortical thickening was shown on T1-weighted images, and a sharp boundary between gray and white matter was usually visible (Figs 1 and 4), although gray-white matter contrast was reduced in more severe cases. Heavily T1-weighted (8000/68; inversion time, 400 ms) inversion recovery images were obtained in 15 cases, and decreased signal intensity, not visible on the T1-weighted fast spoiled gradient-recalled volume acquisition images, was seen within the ipsilateral temporal cortex in two cases (Fig 4).
Fig 2.
Coronal view fast spin-echo T2-weighted images (2975/98) of an 11-year-old female patient who had experienced complex partial seizures since the age of 4 years after prolonged febrile convulsions during infancy. At age 12 years, the patient underwent a temporal lobectomy. Histologic examination showed hippocampal sclerosis, with no dysplasia in the temporal specimen.
A and B, Subtle asymmetry of signal intensity in the white matter of the left temporal pole compared with the right (arrows) is shown, with apparent shrinkage of the white matter due to the slightly increased white matter signal intensity. The appearances on coronal view T1-weighted fast spoiled gradient-recalled volume acquisition images were normal.
C, More posteriorly, right hippocampal sclerosis is shown (arrow), with normal signal intensity within posterior right temporal white matter.
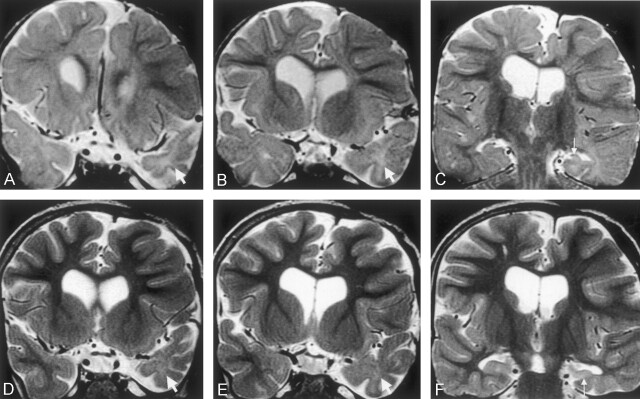
Fig 3.
Coronal view T2-weighted fast spin-echo images (2975/98) of a male patient who experienced onset of epilepsy at age 4 months, soon after drainage of a large left frontal subdural empyema. Clusters of complex partial seizures occurred every week during the first year of epilepsy.
A and B, Images obtained when the patient was 18 months old. An immature appearance persists in both temporal poles, but the ipsilateral left temporal pole is small (arrows), with abnormal increased white matter signal intensity.
C, Image obtained when the patient was 18 months old. Left hippocampal sclerosis with volume loss and increased signal intensity is shown (arrow). Note also the abnormal increased signal intensity in the temporal lobe white matter, compared with the delayed myelination seen elsewhere.
D and E, Images obtained at similar section positions when the patient was 5 years old. The myelination pattern in the frontal lobes and right temporal lobes is now mature. The left temporal pole is atrophic, and the ipsilateral left temporal white matter is now of similar signal intensity to gray matter, although still slightly hyperintense (arrows).
F, Image obtained when the patient was 5 years old. Typical left hippocampal sclerosis is shown (arrow), with a slight increase in white matter signal intensity on the left compared with the right on this more posterior section. Atrophy of the parahippocampal and fusiform gyri can be seen.
MR imaging was performed between 0 and 14 years after epilepsy onset (mean time interval, 6 years). Two patients with anterior temporal changes were imaged within a month of seizure onset. Thirteen patients (seven with and six without anterior temporal changes) underwent follow-up MR imaging at least a year after their initial examinations. All patients with anterior temporal changes on their initial images had these confirmed by subsequent imaging. We did not observe the development of anterior temporal changes on follow-up images of any patient. When anterior temporal changes were present on the initial images, the appearance did not appear to worsen on follow-up images.
Clinical Findings
The clinical findings for patients with hippocampal sclerosis with and without anterior temporal changes are summarized in Table 1. The mean age and sex ratios of the two groups were the same, although a larger proportion of boys had anterior temporal changes. Patients with anterior temporal changes were younger at onset of epilepsy (mean age, 4.3 years), compared with those with no anterior temporal changes (mean age, 6.7 years) (P < .01, Student’s t test). Ten (32%) of the patients with anterior temporal changes experienced onset of epilepsy before 2 years of age, whereas none of the patients without anterior temporal changes experienced such early onset of epilepsy. Fifty-one patients had temporal lobe epilepsy with complex partial seizures. Two patients did not have epilepsy at the time of MR imaging: one patient with anterior temporal changes was investigated for developmental delay and microcephaly but was lost to follow-up after her MR imaging examination, and the other without anterior temporal changes was investigated for events that were eventually thought to be pseudoseizures and had not developed typical epilepsy as of the time of this writing. One additional patient without anterior temporal changes was referred with epilepsy, but no details of seizures were available because of minimal clinical records.
TABLE 1:
Clinical findings for 54 children with hippocampal sclerosis, with and without anterior temporal changes
| Clinical Finding n=54 | AT Signal Changes n = 31 | No AT Signal Changes n=23 | |
|---|---|---|---|
| Mean age at onset of epilepsy (years ±SD) | 4.3 (±2.8) | 6.7 (±3.6) | P < .01 |
| Age at previous cerebral insult (years ±SD) | 0.9 (±0.7) | 3.2 (±3.6) | P < .03 |
| Duration of epilepsy (years ±SD)* | 6.9 (±4.1) | 4.4 (±4.5) | P < .04 |
| Mean number of seizures (±SD)* | 1730 (±3730) | 325 (±480) | P < .05 |
| Number treated surgically (%)* | 19 (61%) | 5 (22%) | P < .004 |
| Mean age at time of MR imaging (years ±SD) | 11.2 (±4.2) | 11.4 (±4.6) | ns |
| Gender (male:female) | 15:16 | 8:15 | ns |
| Number with previous cerebral insult | 27 | 17 | ns |
| Time from insult to epilepsy onset (years ±SD) | 3.1 (±2.8) | 3.9 (±3.5) | ns |
| Overall seizure frequency (sz/wk ±SD) | 7 (±15.8) | 3 (±6.2) | ns |
| Seizure frequency before MR imaging (sz/wk ±SD) | 4.6 (±7.6) | 4.4 (±8.7) | ns |
| Number with previous febrile convulsions (%) | 15 (48%) | 9 (39%) | ns |
| Number with family history of epilepsy (%) | 10 (32%) | 7 (30%) | ns |
Note.—AT indicates anterior temporal; sz, seizures; wk, week; ns, not significant.
Dependent on the age at onset of epilepsy.
An antecedent cerebral insult was present in 27 (87%) patients with anterior temporal changes and in 17 (74%) with no anterior temporal changes (P < .6, χ2 test) (Table 1). Patients with anterior temporal changes were younger at the time of antecedent insult (mean age, 0.9 years) than children without anterior temporal changes (mean age, 3.2 years) (P < .03, Student’s t test). The type of insults varied slightly between the groups (Table 2), with more cases of previous febrile convulsions, meningitis, and early ischemic or hypoxic events in children with anterior temporal changes. In contrast, slightly more cases of previous head injury (three patients with coma and intensive care admission) existed among those with no anterior temporal changes. Both groups had similar numbers with previous febrile convulsions (P < .5, χ2 test) and family history of epilepsy (P < .85, χ2 test) (Table 1). Seizures at the time of early insult were slightly more common in patients with anterior temporal changes, occurring in 22 (81%) of 27 patients with anterior temporal changes and in 11 (65%) of 17 patients without anterior temporal changes (P < .21) (Table 2).
TABLE 2:
Type of antecedent cerebral insult with and without anterior temporal changes
| Type of Antecedent Insult n=44 | AT Signal Changes n = 27 | No AT Signal Changes n=17 |
|---|---|---|
| Febrile convulsions | 13 (13)* | 5 (5) |
| Meningitis | 6 (6) | 1 (1) |
| Encephalitis | 1 (0) | 2 (2) |
| Early ischemic/hypoxic/hypoglycemic event(s) | 6 (2) | 3 (1) |
| Head injury | 3 (2) | |
| Other | 1 (1) | 3 (1) |
| Subdural empyema | 1 (1) | |
| Cavernoma complicated by hemorrhage | 1 (1) | |
| Hypertensive encephalopathy | 1 (0) | |
| Leukemia and encephalopathy | 1 (0) |
Note.—AT indicates anterior temporal.
Numbers in parentheses indicate the number of patients with seizures during the illness.
Overall, patients with anterior temporal changes were more likely to have either epilepsy onset or a previous cerebral insult before age 2 years than patients with no anterior temporal changes (P < .0009, χ2 test). Only four of 31 patients with anterior temporal changes did not have epilepsy onset or an antecedent cerebral insult before 2 years (one temporal ganglioglioma and epilepsy onset at 6 years, one developmental delay without seizures, one meningitis and epilepsy onset at age 2.8 years, and one without antecedent event and temporal lobe epilepsy onset at age 7.3 years). Conversely, nine of 23 patients without anterior temporal changes had an event before age 2 years (four febrile convulsions, one previous head injury, one encephalitis, two neonatal ischemic events, and one intracerebral hemorrhage and multiple cavernomas), eight of these with seizures.
Patients with anterior temporal changes had a longer duration of epilepsy at the time of their MR imaging examination (P < .04, Student’s t test) (Table 1) and a greater overall number of seizures (P < .05, Student’s t test), reflecting the earlier age at onset of epilepsy in this group. Imaging revealed typical anterior temporal changes for four patients with mild epilepsy (<40 seizures) and one with no epilepsy. The estimated mean seizure frequency was similar for both groups, both in the 6 months before MR imaging (P < .93, Student’s t test) and when averaged over the duration of epilepsy (P < .19, Student’s t test). The length of the silent period between a previous cerebral insult and the onset of epilepsy was the same in both groups (P < .43, Student’s t test) (Table 1).
Surgery was performed in 24 patients. The indication for surgery was recurrent seizures that were resistant to anti-epileptic medication and had significant impact on the life of the child and family, as determined by our comprehensive children’s epilepsy program. Nineteen patients with anterior temporal changes were treated surgically with anterior temporal lobectomy, compared with five patients with no anterior temporal changes (P < .004, χ2 test) (Table 1). Histologic confirmation of hippocampal sclerosis was obtained for all 24 operated patients. Focal dysplasia was not revealed by histology, but the anterior temporal specimens were not sufficient for detailed review.
Discussion
Our results indicate that ipsilateral anterior temporal changes occur early during the course of temporal lobe epilepsy associated with hippocampal sclerosis. The prevalence of this appearance in pediatric cases of hippocampal sclerosis is similar to that in adult cases (3), with a similar association between anterior temporal changes and ipsilateral anterior temporal atrophy. When MR imaging is performed after a mature myelin pattern has developed, the appearances in children are identical to those seen in adults, with similar signal intensity seen in gray and white matter on T2-weighted images and resultant loss of gray-white matter definition (Figs 1 and 2). In two patients examined before development of a mature cerebral myelination pattern, increased signal intensity was shown within ipsilateral white matter on T2-weighted images (Figs 3 and 4). On the follow-up images of one of those patients, the white matter hyperintensity became less marked (Fig 3), closer to the appearance seen on images of older children and adults (1, 2). The cause for this initial hyperintensity is unknown but could be analogous to the hippocampal hyperintensity reported in some cases of developing hippocampal sclerosis, thought to represent an early edematous phase before hippocampal volume loss occurs (13–15).
We found that anterior temporal changes are associated with very early onset of epilepsy and early cerebral insult, typically occurring before the age of 2 years. Most patients with anterior temporal changes had either onset of epilepsy or other antecedent cerebral insult before the age of 2 years, and most of the antecedent insults involved seizures. Similar associations have not been conclusively found in adult cases (2), although Choi et al (4) found that adults with anterior temporal changes had earlier onset of epilepsy. This may be because details of the change early in the patients’ history are often more vague when obtained in adulthood and early details not well remembered. We suggest that a cerebral event such as onset of epilepsy or other seizure-related insult occurring before 2 years of age may be an important factor causing these changes to develop.
We do not think that anterior temporal changes per se cause early onset of epilepsy; this would not account for the difference in acquired antecedent insults. On the other hand, we have found no evidence to support the hypothesis that cumulative damage from repeated seizures in the course of chronic temporal lobe epilepsy causes anterior temporal changes. In addition to the similar prevalence in children and adults, anterior temporal changes were observed in mild cases of epilepsy and in one case without epilepsy. We observed anterior temporal changes within a month after the onset of epilepsy, and the severity did not appear to increase with time based on follow-up MR images. Although children with anterior temporal changes experienced longer duration of epilepsy and greater total number of seizures than those without anterior temporal changes, this probably reflected the earlier onset of epilepsy; the overall seizure frequency was not different between the two groups. Similarly, although children with anterior temporal changes were more often treated surgically, this most probably reflected the longer duration of epilepsy and cumulative seizures during this period. Thus, we found no evidence that epilepsy is more severe in patients with anterior temporal changes, in concordance with previous studies of adult cases (2, 4). If seizures are the cause, anterior temporal changes probably develop at or soon after the onset of seizures.
An alternative view presented by some authors, which we think to be incorrect in all except a small minority of cases, is that anterior temporal changes represent cortical dysplasia and therefore predate hippocampal sclerosis (6, 9). Three independent studies have been conducted, comprised of approximately 80 surgically treated patients with anterior temporal changes, that have found only subtle histologic abnormalities in resected specimens, with no cortical dysplasia (2–4). In addition, the MR imaging appearances are atypical for focal cortical dysplasia with less hyperintensity on T2-weighted images (10) and a sharp boundary often maintained between gray and white matter without cortical thickening on T1-weighted images. We found one patient with a temporal ganglioglioma that may have contributed to the anterior temporal appearances, but based on previous studies (2–4), we think that cortical dysplasia and foreign tissue lesions only rarely cause this appearance in hippocampal sclerosis. In their study of 18 patients with temporal lobe epilepsy with anterior temporal changes, Choi et al (4) found increased numbers of isolated ectopic neurons in the white matter but conceded that these subtle abnormalities were unlikely to constitute the entire explanation. Other authors have proposed that architectural cortical dyslamination is the cause (7, 8) but have not explained how these intracortical changes can produce diffuse white matter signal intensity abnormality as the predominant MR imaging finding (Figs 1–4). Overall, because anterior temporal changes are often dramatic, with only subtle identified histologic abnormalities, it seems likely that an important component of the underlying abnormality is a lesion that is difficult to identify histologically, such as an alteration of myelin or an alteration in tissue water content (1–3). Although there are many myelinated fibers within the cortex, the presence of cortical signal intensity abnormality in some cases (2) (Fig 4) may indicate pathologic abnormality in addition to abnormality of myelin.
If abnormality of anterior temporal myelin is the cause of the white matter component of anterior temporal changes, when might this abnormality be expected to occur? It is thought that once the myelin sheath has formed, it is relatively insensitive to insult and that the period of active myelination is the most vulnerable to injury (16, 17). Myelination commences around term and is virtually complete by 2 years (17, 18), with most activity occurring during the first 8 months after term (17). It proceeds from central areas toward the poles, reaching the tips of the frontal and temporal lobes last (17). Considerable normal variation exists (17, 18) with mature myelin found postmortem in the temporal poles of 50% of infants by 82 weeks after term (17), but maturation of the subcortical association fibers of the temporal poles continues after 2 years (18). Therefore, myelin in the temporal poles may be vulnerable to injury for up to 2 years or beyond.
Our finding that most children whose images show anterior temporal changes have experienced epilepsy onset or other seizure-associated insult during the vulnerable period of active myelination suggests that the seizures or injury could interrupt or prevent normal anterior temporal myelination. It is not known whether seizures can disturb myelination in humans (16), but in immature rats with repeated seizures, defects in lipid metabolism and myelin accumulation have been shown, with the most severe defects found when seizures occurred early, during the phase of glial proliferation (19–21). We suggest that the lack of normal gray-white matter definition on T2-weighted images showing anterior temporal changes may represent abnormal persistence of an immature myelin pattern in the temporal poles. However, if abnormal myelin is the explanation, it is likely that other unidentified factors such as genetic susceptibility or severity of injury are important, because not all children with early injury develop anterior temporal changes and vice versa.
Because anterior temporal changes have been observed in cases of intractable temporal lobe epilepsy without hippocampal sclerosis, albeit infrequently (2), it is likely that they do not develop as a consequence of hippocampal sclerosis. However, the pathogenesis of anterior temporal changes and hippocampal sclerosis may overlap, particularly because they are always ipsilateral to the hippocampal sclerosis in this and other reported series. Although the cause of hippocampal sclerosis is not known, recent cytoarchitectural studies of anterior temporal specimens have suggested that hippocampal sclerosis may be partly due to abnormal mesial temporal organization (22). Cajal-Retzius cells, a specific early developmental population of interneurons, can persist abnormally until after childhood in the hippocampus and temporal neocortex of patients with hippocampal sclerosis (22, 23). Also, features suggesting neurogenesis can be found in resected anterior temporal specimens, either indicating ongoing postnatal neurogenesis or an abnormally long period of maturation (22). For these reasons, Blumcke et al (22) suggest that hippocampal sclerosis may have early onset, with persistent focal immaturity or delayed maturation as an important part of the pathogenesis. We speculate that the pathogenesis of anterior temporal changes may partly mirror the pathogenesis of hippocampal sclerosis and also represent persistent immaturity. The subtle cortical dyslamination found in some anterior temporal specimens (7, 8) suggests maldevelopment, but these small histopathologic changes do not necessarily occur before birth (22, 24, 25). Acquired injury during the first years of life, while the immature temporal lobe continues to develop (17, 18), could give rise to all these “developmental” abnormalities and lead to the persistent appearance of an immature anterior temporal lobe.
Conclusion
We found that ipsilateral anterior temporal changes associated with hippocampal sclerosis can be seen on images obtained during early childhood, with prevalence and MR imaging appearances similar to those in adult cases. They are part of the spectrum of hippocampal sclerosis and may indicate widespread abnormality affecting the temporal lobe. Further large studies of new onset and intractable temporal lobe epilepsy, with and without hippocampal sclerosis, may help to determine these relationships. We found that anterior temporal changes are associated with early onset of epilepsy and early cerebral insult that usually involves seizures, typically occurring before the age of 2 years. We think that the lack of gray-white matter definition on T2-weighted images represents an abnormal persistent immature appearance, including an abnormality of myelin or myelination, and may be the result of seizure-related injury to the anterior temporal lobe during maturation.
Footnotes
This work was supported by a National Health and Medical Research Council Program Grant (to G.D.J.).
References
- 1.Meiners LC, van Gils A, Jansen GH, et al. Temporal lobe epilepsy: the various MR appearances of histologically proven mesial temporal sclerosis. AJNR Am J Neuroradiol 1994;15:1547–1555 [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell LA, Jackson GD, Kalnins RM, et al. Anterior temporal abnormality in temporal lobe epilepsy: a quantitative MRI and histopathologic study. Neurology 1999;52:327–336 [DOI] [PubMed] [Google Scholar]
- 3.Meiners LC, Witkamp TD, de Kort GA, et al. Relevance of temporal lobe white matter changes in hippocampal sclerosis: magnetic resonance imaging and histology. Invest Radiol 1999;34:38–45 [DOI] [PubMed] [Google Scholar]
- 4.Choi D, Na DG, Byun HS, et al. White-matter change in mesial temporal sclerosis: correlation of MRI with PET, pathology, and clinical features. Epilepsia 1999;40:1634–1641 [DOI] [PubMed] [Google Scholar]
- 5.Moran NF, Lemieux L, Kitchen ND, Fish DR, Shorvon SD. Extrahippocampal temporal lobe atrophy in temporal lobe epilepsy and mesial temporal sclerosis. Brain 2001;124:167–175 [DOI] [PubMed] [Google Scholar]
- 6.Kuzniecky R, de la Sayette V, Ethier R, et al. Magnetic resonance imaging in temporal lobe epilepsy: pathological correlations. Ann Neurol 1987;22:341–347 [DOI] [PubMed] [Google Scholar]
- 7.Tassi L, Colombo N, Garbelli R, et al. Focal cortical dysplasia: neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain 2002;125:1719–1732 [DOI] [PubMed] [Google Scholar]
- 8.Mohamed A, Wyllie E, Ruggieri P, et al. Temporal lobe epilepsy due to hippocampal sclerosis in pediatric candidates for epilepsy surgery. Neurology 2001;56:1643–1649 [DOI] [PubMed] [Google Scholar]
- 9.Ho SS, Kuzniecky RI, Gilliam F, Faught E, Morawetz R. Temporal lobe developmental malformations and epilepsy: dual pathology and bilateral hippocampal abnormalities. Neurology 1998;50:748–754 [DOI] [PubMed] [Google Scholar]
- 10.Ryvlin P, Coste S, Hermier M, Mauguiere F. Temporal pole MRI abnormalities in temporal lobe epilepsy. Epileptic Disord 2002;4[suppl 1]:S33–S39 [PubMed] [Google Scholar]
- 11.Jackson GD, Berkovic SF, Tress BM, Kalnins RM, Fabinyi GC, Bladin PF. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology 1990;40:1869–1875 [DOI] [PubMed] [Google Scholar]
- 12.Jackson GD, Berkovic SF, Duncan JS, Connelly A. Optimizing the diagnosis of hippocampal sclerosis using MR imaging. AJNR Am J Neuroradiol 1993;14:753–762 [PMC free article] [PubMed] [Google Scholar]
- 13.VanLandingham KE, Heinz ER, Cavazos JE, Lewis DV. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol 1998;43:413–426 [DOI] [PubMed] [Google Scholar]
- 14.Perez ER, Maeder P, Villemure KM, Vischer VC, Villemure JG, Deonna T. Acquired hippocampal damage after temporal lobe seizures in 2 infants. Ann Neurol 2000;48:384–387 [PubMed] [Google Scholar]
- 15.Jackson GD, Chambers BR, Berkovic SF. Hippocampal sclerosis: development in adult life. Dev Neurosci 1999;21:207–214 [DOI] [PubMed] [Google Scholar]
- 16.Volpe JJ. Neurology of the Newborn. 4th ed. Philadelphia, W.B. Saunders Company;2001. :83–99, 178–211
- 17.Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy: II. patterns of myelination in autopsied infants. J Neuropathol Exp Neurol1988;47:217–234 [DOI] [PubMed] [Google Scholar]
- 18.Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy: I. an autopsy study of myelination. J Neuropathol Exp Neurol 1987;46:283–301 [DOI] [PubMed] [Google Scholar]
- 19.Wasterlain CG. Recurrent seizures in the developing brain are harmful. Epilepsia 1997;38:728–734 [DOI] [PubMed] [Google Scholar]
- 20.Wasterlain CG, Shirasaka Y. Seizures, brain damage and brain development. Brain Dev 1994;16:279–295 [DOI] [PubMed] [Google Scholar]
- 21.Dwyer BE, Wasterlain CG. Electroconvulsive seizures in the immature rat adversely affect myelin accumulation. Exp Neurol 1982;78:616–628 [DOI] [PubMed] [Google Scholar]
- 22.Blumcke I, Thom M, Wiestler OD. Ammon’s horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol 2002;12:199–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thom M, Sisodiya S, Harkness W, Scaravilli F. Microdysgenesis in temporal lobe epilepsy: a quantitative and immunohistochemical study of white matter neurones. Brain 2001;124:2299–2309 [DOI] [PubMed] [Google Scholar]
- 24.Mathern GW, Babb TL, Mischel PS, et al. Childhood generalized and mesial temporal epilepsies demonstrate different amounts and patterns of hippocampal neuron loss and mossy fibre synaptic reorganization. Brain 1996;119:965–987 [DOI] [PubMed] [Google Scholar]
- 25.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 1997;17:3727–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]