Abstract
BACKGROUND AND PURPOSE: Loss of neurons results in a relative increase in extracellular space that may lead to altered apparent diffusion coefficient (ADC) values in the hippocampi of patients with seizures. Our purpose was to determine if ADC values along the long axis of hippocampi are useful in evaluating patients with partial complex seizures.
METHODS: Hippocampi of 23 patients with partial complex seizures and 25 healthy volunteers were evaluated with MR imaging and ADC maps. MR images were evaluated for loss of volume and/or high signal intensity on T2-weighted images and compared with ADC maps. ADCs were compared between patients and controls, as were ADCs along the length of each hippocampus. Mean and SDs were obtained for each measurement, and level of significance was determined (P < .05). The relationship between clinical lateralization and MR imaging and ADCs was studied.
RESULTS: No significant variations were found in the ADCs in controls (side to side and along hippocampi). In patients, abnormalities were seen with MR imaging alone in 16, with ADC in 14, and with both in 21. Of 23 hippocampi with an abnormal MR appearance, 14 had abnormal ADCs. Nine hippocampi with a normal MR appearance had abnormal ADCs. Normal MR appearance and ADCs were seen in 13 hippocampi. Most abnormal ADCs were seen in the anterior aspect of the hippocampi. All differences were statistically significant. Of 19 patients who underwent clinical testing, unequivocal lateralization was established in 10. Concordance between clinical tests and MR imaging, ADC, and MR imaging plus ADC was found in five, five, and seven patients, respectively.
CONCLUSION: Visual assessment was better than ADCs alone for detection of abnormal hippocampi. MR imaging plus ADCs was better than either technique alone. ADCs may be abnormal when MR images are unremarkable. Concordance with clinical lateralization was better when MR imaging and ADC were jointly evaluated than when either technique was evaluated separately.
Complex partial seizures commonly arise from the mesial basal structures of the temporal lobes and from the frontal neocortex. A history of febrile seizures or encephalitis during infancy is frequent in mesial temporal sclerosis (MTS) (1, 2). Partial complex seizures of temporal lobe origin usually manifest with oral-alimentary automatisms, repetitive hand movements, or looking around (3, 4). MTS is a frequent cause of intractable temporal lobe epilepsy, and the most common MR imaging findings are hippocampal atrophy and/or high signal intensity on T2-weighted images. Quantitative MR imaging such as hippocampal volumetry and T2 relaxometry may help detect up to 80% of MTS cases, and in the remaining 20% these studies are normal (5, 6). MR spectroscopy shows abnormalities in greater than 90% of patients with hippocampal sclerosis but also requires special expertise. These abnormalities mainly include low N-acetylaspartate and occasionally the presence of lactate, which may be related to recent seizures.
Because diffusion-weighted (DW) imaging is readily available, easy to perform, and relatively easy to interpret, it is a desirable technique for evaluating patients with seizure. DW imaging allows for evaluation of differences in the extracellular space through variations of molecular water mobility at a microscopic level. Diffusion of water molecules depends on structures within tissues (intracellular organelles, macromolecules, membranes, etc), viscosity, temperature, degree of myelination, fiber packing and shape, and cell types present (7). Contraction of the extracellular spaces during epileptic activity owing to a water flux into cells at the area of maximum neuronal activity may explain low hippocampal apparent diffusion coefficient (ADC) values seen in MTS (8–12). Spreading depression due to mechanical, electrical, or chemical stimuli secondary to seizures produces membrane depolarization of both neurons and glia, which results in a spontaneously reversible failure of ion homeostasis. This results in an energy deficiency that leads to a failure of the sodium-potassium pump, causing an influx of water into the cells of the gray matter (8, 11, 12).
Our purpose in this study was to assess the utility of hippocampal ADC measurements in patients with known temporal lobe seizures.
Methods
After obtaining approval by our institutional review board, we studied 25 healthy individuals (age range, 1–46 years; mean age, 24.36 years; not matched for handedness) and 23 patients (age range, 3–43 years; mean age, 21.47 years) with complex partial seizures who underwent MR imaging during their seizure work-up. All patients had temporal lobe seizures based on clinical examination, electroencephalogram (EEG), or videoelectroencephalogram (VEEG).
Brain MR images were obtained with a 1.5-T system in both the control subjects and patients. Imaging protocol consisted of sagittal 5-mm-thick T1-weighted images (470/12 [TR/TE]); coronal 1.5-mm (no intersection gap) 3D T1-weighted gradient-echo images (1900/4.4, flip angle 8°) through the entire brain; coronal 2-mm-thick fluid-attenuated inversion-recovery (FLAIR) images (8600/108/2400 [TR/TE/TI]); coronal 3-mm-thick T2-weighted images (6860/125) angled perpendicular to the long axis of the hippocampus (from the amigdala to the tail); angled axial 5-mm-thick T2-weighted images (6860/125); and angled axial 5-mm FLAIR images (8600/108/2400) oriented along the long axis of the hippocampi.
Two neuroradiologists (A.L., M.C.) blinded to clinical history evaluated all studies qualitatively for the presence of unilateral or bilateral hippocampal atrophy and/or hippocampal hyperintensity on T1-weighted, T2-weighted, and FLAIR images. Differences in opinions were resolved by consensus.
DW images were obtained angled along the long axis of the hippocampi, with b values of 0, 500, and 1000 s/mm2 (180/122, 5-mm section thickness), and ADC maps (matrix 128 × 128) were generated by using the commercial software provided by the manufacturer. On the axial ADC maps, we divided each hippocampus into arbitrary four segments and manually placed circular regions of interest (ROIs) of similar size (35–55 pixels) roughly corresponding to the head (segment A), body (segments B and C), and tail (segment D) of the hippocampus (Fig 1). The ROIs were drawn to include as much of the hippocampal tissues as possible while avoiding the adjacent CSF-containing spaces, to decrease partial volume effects from fluid. To exclude variations in ADC values related to age differences, we divided the control group into those aged 20 years or younger and those between 21 and 50 years of age. Mean and SDs were obtained in each ROI (for both left and right hippocampi). The level of significance for the analysis was a P value less than .05. In the patient group, ADC values plus or minus two SDs from the normal range were considered abnormal. We then compared the ADC values with the appearance of the hippocampi on MR images. Also, we assessed the number and location of ROIs showing abnormal ADC values within each hippocampus.
Fig 1.
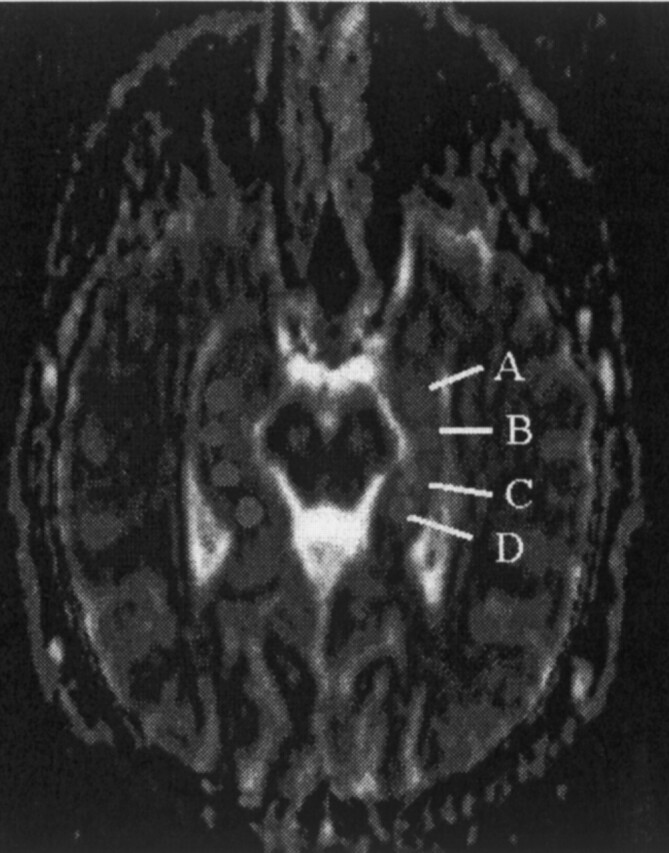
Location of ADC measurements. Angled axial section from ADC map obtained along the long axis of the hippocampi shows placement and size of the ROIs: head (segment A), body (segments B and C), and tail (segment D) of the hippocampi.
Information regarding lateralization of seizure foci was available in 19 patients and was obtained from review of the electroencephalogram (n = 11), video electroencephalogram (n = 7), single photon emission tomograms (SPECT, n = 8), positron emission tomograms (PET, n = 3), and proton MR spectroscopic images (n = 9). One or more of these studies had to show unequivocal and concordant lateralization to be considered as proof of diagnosis.
Results
In the control group, no significant variations of the ADC hippocampal values were found for the average mean between left and right hippocampi or along their axis regardless of age (Table 1). In all of the control subjects, the MR appearance of the hippocampi was judged to be normal.
TABLE 1:
ADC values in control subjects
| Group | Left Segment |
Right Segment |
||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | |
| Aged 0–20 y | 93.67 ± 6.14 | 92.03 ± 7.49 | 91.39 ± 0.47 | 90.23 ± 13.81 | 96.32 ± 5.24 | 91.75 ± 3.71 | 93.33 ± 7.54 | 88.57 ± 11.23 |
| Aged 21–50 y | 94.68 ± 10.4 | 94.97 ± 8.78 | 95.51 ± 10.22 | 91.92 ± 11.04 | 91.44 ± 13.13 | 92.2 ± 10 | 93.18 ± 11.61 | 88.92 ± 12.28 |
Note.—Data are mean values ± SD (1 × 10−5 mm2/s). A indicates head of hippocampus; B and C, body; D, tail.
In the patients with temporal lobe seizures, abnormalities were seen with MR imaging alone in 16 patients, with ADC in 14, and with MR imaging plus ADC in 21 patients. ADC values in each patient are listed in Table 2. The relationship between the MR imaging appearance of the hippocampi and their ADC values is shown in Table 3. In the patient group, 23 of 46 hippocampi were abnormal by MR imaging and 14 showed high ADC values. When we assessed each hippocampus individually, seven had an abnormal MR appearance with significantly elevated ADC values. Normal MR appearance with abnormal ADC values was found in nine hippocampi. Of these, six hippocampi showed high ADC and three low ADC values. Seventeen hippocampi had an abnormal MR appearance but normal ADC values. Normal MR appearance and normal ADC values were seen in 13 hippocampi. Only one hippocampus showed abnormal ADC values in all four segments measured. Six had high ADC values in two segments (four in segments A and B and two in segments B and C). Nine had high ADC values in only one segment (six in segment B, two in segment A, and one in segment D). Of the 16 hippocampi with abnormal ADC values, 14 had abnormalities in either segment A or B (thus most abnormal ADC values were found in the anterior hippocampal regions). All the differences found in the ADC values in the patient group were statistically significant.
TABLE 2:
ADC values in patients
| Patient, No./Age (y) | Left Segment |
Right Segment |
||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | |
| 1/33 | 94.3 | 91.43 | 92.21 | 78.78 | 92.18 | 85.97 | 83.59 | 80.57 |
| 2/19 | 109.81* | 111.18* | 96.83 | 74.81 | 94.22 | 93.75 | 85.45 | 83.38 |
| 3/5 | 90.22 | 82.31 | 89.7 | 81.71 | 97 | 92.26 | 87.02 | 85.17 |
| 4/26 | 108.9 | 107.41 | 106.28 | 91.75 | 99.18 | 104.41 | 99.62 | 97.96 |
| 5/30 | 96.12 | 91.72 | 98.5 | 92.48 | 133.45* | 124* | 105.27 | 105.14 |
| 6/26 | 101.64 | 92.32 | 96.21 | 91.38 | 102.09 | 119* | 102.59 | 100.41 |
| 7/3 | 95.59 | 101.02 | 95.24 | 96.64 | 94.54 | 94.67 | 96.06 | 95.2 |
| 8/13 | 118.38* | 113.16* | 99.53 | 95.7 | 103.54 | 118.12* | 112.2* | 100.93 |
| 9/19 | 94.03 | 97.51 | 84.81 | 90.63 | 95.29 | 100.91* | 90.53 | 88.78 |
| 10/5 | 92.41 | 87.59 | 80.79 | 78.54 | 87.96 | 83.79* | 87.28 | 85.92 |
| 11/9 | 93.77 | 84.43 | 95.84 | 119.70* | 94.43 | 87.25 | 96.45 | 83.21 |
| 12/20 | 90.31 | 89 | 84.09 | 86.15 | 97.4 | 86.78 | 85.55 | 87.62 |
| 13/29 | 94.9 | 104.92 | 85.81 | 84 | 136.21* | 96.22 | 88.03 | 86.74 |
| 14/9 | 92.55 | 88.44 | 83.65 | 90.83 | 91.86 | 85.73 | 81 | 95.05 |
| 15/11 | 151.93* | 137.81* | 110.34 | 103.76 | 94.5 | 106.68* | 116.84* | 106.48 |
| 16/7 | 100.79 | 102.47 | 83.54 | 78.7 | 101.36 | 103.41* | 85.13 | 94 |
| 17/9 | 91.82 | 85.76 | 91.12 | 80.85 | 89.55 | 79.27* | 85.32 | 84.21 |
| 18/43 | 85.7 | 92.09 | 81.9 | 73.02 | 121.17* | 100 | 81.72 | 78.16 |
| 19/33 | 93.86 | 83.42 | 89.18 | 83.13 | 92.09 | 96.43 | 85.23 | 79.26 |
| 20/26 | 89.62 | 106.23 | 84.57 | 84.4 | 84.15 | 99 | 88 | 95.22 |
| 21/35 | 94.7 | 78.59 | 79.11 | 83.65 | 88.96 | 101.11 | 90.13 | 86.51 |
| 22/42 | 85.4 | 82.05 | 70.06* | 80.58 | 90.42 | 93.95 | 82.79 | 77.8 |
| 23/43 | 120.72* | 115.67* | 127.02* | 115.92* | 100.38 | 86.66 | 86.51 | 78.18 |
Note.—Data are expressed as 1 × 10−5 mm2/s. A indicates head of the hippocampus; B and C, body; D, tail.
*
TABLE 3:
Relationship between MR appearance of hippocampi and ADC values
| Patient No./Age (y)/Sex | MR Appearance | ADC Value |
|---|---|---|
| 1/33/F | Abnormal L | Normal |
| 2/19/M | Normal bi | ↑ LA, LB |
| 3/5/M | Abnormal bi | Normal |
| 4/26/F | Abnormal bi | Normal |
| 5/30/F | Abnormal R | ↑ RA, RB |
| 6/26/M | Abnormal L | ↑ RB |
| 7/3/M | Normal bi | Normal |
| 8/13/F | Abnormal L | ↑ LA, LB, RB, RC |
| 9/19/F | Normal bi | ↑ RB |
| 10/5/F | Normal bi | ↓ RB |
| 11/9/M | Normal bi | ↑ LD |
| 12/20/ | Abnormal R | Normal |
| 13/29/M | Abnormal bi | ↑ RA |
| 14/9/F | Normal bi | Normal |
| 15/11/ | Abnormal L | ↑ LA, LB, RB, RC |
| 16/7/M | Abnormal bi | ↑ RB |
| 17/9/F | Normal bi | ↓ RB |
| 18/43/M | Abnormal R | ↑ RA |
| 19/33/M | Abnormal L | Normal |
| 20/26/ | Abnormal bi | Normal |
| 21/35/F | Abnormal bi | Normal |
| 22/42/M | Abnormal bi | ↓ LC |
| 23/42/M | Abnormal bi | ↑ LA, LB, LC, LD |
L indicates left; R, right; bi, bilateral; A, head of the hippocampus; B and C, body of the hippocampus; D, tail of the hippocampus; ↑, increased; ↓, decreased.
The relationship between lateralization and MR imaging and ADC values was established for each patient. Of the 23 patients evaluated at the time of this report, tests (EEG, VEEG, SPECT, PET, and MR spectroscopy) had been obtained in 19. In nine of 10 patients, unequivocal lateralization (right in three, left in six) was documented (EEG, four patients; VEEG, three; SPECT, three; PET, two; MR spectroscopy, two). In one patient, SPECT showed bilateral abnormalities. Tests were considered nonconclusive in nine patients. Unilateral (n = 4) and bilateral (n = 1) concordance between MR imaging abnormalities and other tests was found in five patients (six hippocampi). Concordance between abnormal ADC values and other tests was found in five patients (five hippocampi). Concordance between abnormal MR imaging plus ADC and other tests was found in seven patients (seven hippocampi). Discordance among clinical tests, MR imaging, and ADC was encountered in only one patient.
Discussion
In our study, MR imaging and ADC values together showed abnormalities in 91% of patients (21 of 23). In seven patients, the side with abnormal MR appearance and ADC values was in concordance with other test results (EEG, VEEG, SPECT, PET, or MR spectroscopy). The presence of increased ADC values and ipsilateral hippocampal atrophy or high signal intensity confirms previous observations (9, 10, 11). Ipsilateral hippocampal atrophy and increased ADC values are the result of neuronal loss. Loss of neurons results in a relative increase of the extracellular compartment, leading to increased diffusion of water in hippocampal sclerosis. Neuronal loss is associated with a long history of epilepsy (13). Gliosis, which is found in chronic injuries, generally has a microcystic component that also contributes to further increased extracellular water movement, resulting in elevation of ADC values. Coexistence of MR imaging abnormalities and ipsilateral ADC changes show that the use of both techniques may be promising in the evaluation of patients with temporal lobe seizures.
Explaining why ADC values were normal in 17 atrophic hippocampi by MR imaging is difficult. A caveat of our investigation is that information regarding the time of each patient’s last seizure was not available. Despite this, we propose that one explanation for a normal ADC value in a visually abnormal hippocampus may be the presence of concomitant chronic and acute changes. Chronic changes result in an increase of the extracellular spaces, whereas acute changes (possibly due to recent seizures) induce water flux into the cells, contracting the extracellular space. Also, the water inside the cells resides in a more complex environment and is able to move less. The summation of these factors may result in a final ADC value that has ‘pseudonormalized’.
We postulate that high ADC values and a normal MR appearance may represent early involvement of the hippocampi that is not obvious on qualitative MR images. Since we did not obtain hippocampal volumetrics, we were not able to comment on small degrees of volume loss, and since at this time we have no long-term follow-up on our patients we do not know which ones will develop hippocampal atrophy. Abnormal ADC values in normal-appearing white matter have been found in patients with multiple sclerosis and aging, suggesting that ADC values may be more sensitive than conventional MR imaging in depicting changes in the brain parenchyma (14, 15). Thus, it is possible that a similar situation may occur early on in patients with temporal lobe seizures.
Abnormally decreased ADC values were found in three hippocampi that were judged to be normal on MR images. Decreased ADC values have been observed after status epilepticus or seizures in animal models and humans, indicating restriction in the mobility of water molecules (8). Since we have no information regarding the timing of our studies with respect to the patients’ seizures, we cannot comment further on this issue but believe that the above comment may explain this finding.
Perhaps the most important caveat in our investigation is the lack of information regarding lateralization in all patients. A variety of tests that included EEG, VEEG, SPECT, PET, and MR spectroscopy were performed in 19 patients, but unfortunately not all tests were performed in all patients. Thus, we assumed lateralization when unequivocal results were present with one or more tests per patient. In nine patients, all of these tests did not permit us to lateralize the abnormal hippocampus because of contradictory or negative results. We do not have histologic proof of MTS in any of our patients. The data for our study were accumulated over a short period of time, and patients had not yet been operated on. Despite this, we believe that our study results are important because when MR imaging and ADC values are used jointly, the possibility of detecting abnormalities increases. In addition, our observations provide an insight regarding structural and functional abnormalities in diseased hippocampi because the ADC measures an intrinsic tissue property and not an MR imaging property (like T1, T2 and magnetization transfer). We cannot exclude the possibility that inclusion of CSF into the ROIs for measuring the ADC may have influenced our results. However, we were extremely careful to avoid this possibility when obtaining our measurements.
Our findings suggest that hippocampal involvement as assessed with ADC may be segmental. In most of our patients, the anterior aspects of the hippocampi showed the most abnormal ADC values. It is known that the degree of involvement in abnormal hippocampi can vary along their lengths (16). Furthermore, most patients will respond favorably to anterior resections only, implying that in most of them it is the anterior hippocampi that are involved (17).
Conclusion
We found that visual assessment with MR imaging was better than ADC alone for detection of abnormal hippocampi. Combining the results of MR imaging and ADC maps performed better than both techniques alone. The ADC value may be abnormal in the presence of a normal MR appearance, and this may be related to early changes. Conversely, the ADC value may be ‘pseudonormal’ in the presence of an abnormal MR appearance. Concordance between clinical lateralization was better when MR images and ADC maps were evaluated jointly than when either of these techniques was evaluated separately. In our patients with temporal lobe seizures, both MR appearance and ADC values were normal in 37%.
References
- 1.Wetjen NM, Cohen-Gadol AA, Maher CO, et al. Frontal lobe epilepsy: diagnosis and surgical treatment. Neurosurg Rev 2002;25:119–138 [DOI] [PubMed] [Google Scholar]
- 2.Chabolla D. Characteristics of the epilepsies. Mayo Clin Proc 2002;77:981–990 [DOI] [PubMed] [Google Scholar]
- 3.Salanova V, Morris HH, Van Ness P, et al. Frontal lobe seizures: electroclinical syndromes. Epilepsia 1995;36:16–24 [DOI] [PubMed] [Google Scholar]
- 4.Kramer U, Rivello JJ, Carmat P, et al. Clinical characteristics of complex partial seizures: a temporal versus a frontal lobe onset. Seizure 1997;6:57–61 [DOI] [PubMed] [Google Scholar]
- 5.Van Paesschen W, Connelly A, Johnson Ch, Duncan JS. The amygdale and intractable temporal lobe epilepsy: a quantitative magnetic resonance imaging study. Neurology 1996;47:1021–1031 [DOI] [PubMed] [Google Scholar]
- 6.Mosewich RK, So EL, O’Brien TJ, et al. Factors predictive of the outcome of frontal lobe epilepsy surgery. Epilepsia 2000;41:843–849 [DOI] [PubMed] [Google Scholar]
- 7.Gray L, MacFall J. Overview of diffusion imaging. Magn Reson Imaging Clin N Am 1998;6:125–138 [PubMed] [Google Scholar]
- 8.Lux HD, Heinemann U, Dietzel I. Ionic changes and alterations in the size of the extracellular space during epileptic activity. Adv Neurol 1986;44:619–639 [PubMed] [Google Scholar]
- 9.Zhong J, Petroff OA, Prichard JW, et al. Changes in water diffusion and relaxation properties of rat cerebrum during status epilepticus. Magn Reson Med 1993;30:241–246 [DOI] [PubMed] [Google Scholar]
- 10.Wieshmann UC, Clark CA, Symms MR, et al. Water diffusion in the human hippocampus in epilepsy. Magn Reson Imaging 1999;17:29–36 [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Majors A, Najm I, et al. Postictal alterations of sodium content and apparent diffusion coefficient in epileptic rat brain induced by kainin acid. Epilepsia 1996;37:1000–1006 [DOI] [PubMed] [Google Scholar]
- 12.Hasegawas Y, Latour LL, Formato JE, et al. Spreading waves of a reduced diffusion coefficient of water in normal and ischemic rat brain. J Cereb Blood Flow Metab 1995;15:179–187 [DOI] [PubMed] [Google Scholar]
- 13.Sagar HJ, Oxbury JM. Hippocampal neuron loss in temporal lobe epilepsy: correlation with early childhood convulsion. Ann Neurol 1987;22:334–340 [DOI] [PubMed] [Google Scholar]
- 14.Nusbaum AO, Tang CY, Buchsbaum MS, et al. Regional and global changes in cerebral diffusion with normal aging. AJNR Am J Neuroradiol 2001;22:136–142 [PMC free article] [PubMed] [Google Scholar]
- 15.Horsfield MA, Lai M, Webb SL, et al. Apparent diffusion coefficients in benign and secondary progressive multiple sclerosis by nuclear magnetic resonance. Magn Reson Med 1996;36:393–400 [DOI] [PubMed] [Google Scholar]
- 16.Babb TL, Brown WJ, Pretorius J, et al. Temporal lobe volumetric cell densities in temporal lobe epilepsy. Epilepsia 1984;25:729–740 [DOI] [PubMed] [Google Scholar]
- 17.O’Connor WM, Masukawa L, Freese A, et al. Hippocampal cell distributions in temporal lobe epilepsy: a comparison between patients with and without an early risk factor. Epilepsia 1996;37:440–449 [DOI] [PubMed] [Google Scholar]


