Abstract
Summary: Ossifying fibroma is a rare benign neoplasm that usually affects mandibular and maxillary bones. In this report, we present a case of sinonasal ossifying fibroma with fluid-fluid levels and posterior extension toward the torus tubarius on MR images.
Ossifying fibroma is a rare benign fibrous lesion that was first introduced in the literature by Montgomery (1). It mainly develops in the mandible, where it is usually slow growing and asymptomatic, whereas in the midface and paranasal sinuses, it is more aggressive (2–4). The presence of fluid-fluid levels in a bone tumor strongly suggests an aneurysmal bone cyst, although fluid-fluid levels are also described in some bone and soft-tissue tumors (5, 6). In a previous report (7), fluid-fluid levels were described in an ossifying fibroma appearing as an ethmoid mucocele.
In our case, a sinonasal ossifying fibroma involved the right side of nasal cavity and extended into the right maxillary sinus, periorbital fat, anterior cranial cavity, and right torus tubarius. The nasal-cavity component of the mass had multiple fluid-fluid levels, as depicted at MR imaging.
Knowledge of the possible MR imaging characteristics of this rare tumor helps in the differential diagnosis of tumors involving the head and neck region.
Case Report
A 24-year-old female patient presented with headache, swelling of the right eyelid, and a sensation of numbness in the right eye. The patient’s history and family history were unremarkable. On physical examination, right exopthalmos was observed, along with a polypoid mass that filled the right nasal cavity.
CT revealed an attenuating mass predominantly located on the right side of nasal cavity. The mass extended to the base of frontal lobe and to the right orbita.
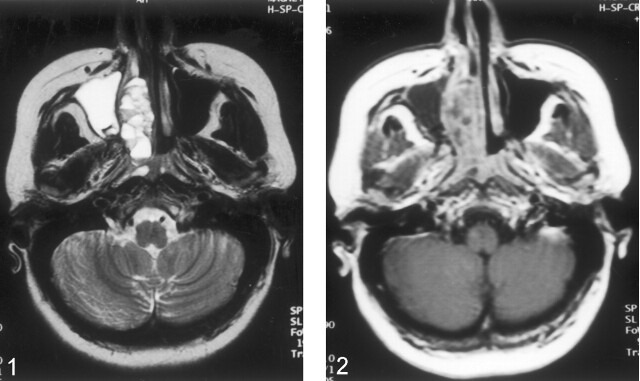
MR examination was performed with a 1.5-T MR unit (Magnetom Vision; Siemens Medical Systems, Erlangen, Germany). T1-weighted spin-echo images (TR/TE, 635/14) and T2-weighted turbo spin-echo images (5000/99) were obtained in the coronal and axial planes (Fig 1). A lobulated mass involving the right nasal cavity and ethmoid sinus extended toward the right maxillary sinus laterally and to the periorbital area superolaterally. The mass caused compression of the right optic nerve and extended to the right torus tubarius posteriorly. The lesion also extended into the anterior cranial fossa. On T1-weighted images, the mass was isointense to hyperintense relative to the neighboring skeletal muscles. On T2-weighted images, the lesion was mainly hyperintense, with fluid-fluid levels in its nasal component. After the administration of IV contrast material (Gadolinium dimeglumine, 0.1 mmol/kg), the periphery and septa of the lesions were enhancing (Fig 2).
Fig 1.
Transverse T2-weighted MR image demonstrates the lesion in the right side of nasal cavity and fluid-fluid levels within the lesion.
Fig 2.
Transverse gadolinium-enhanced T1-weighted MR image reveals enhancement of the periphery and septa of the mass.
Because the mass was located predominantly on the right side of nasal cavity and attached to the nasal septum, as determined during intraoperative evaluation, it was accepted as arising from the nasal cavity. The entire lesion was grossly excised, and the orbital roof was reconstructed by using a bone graft. Pathologic examination revealed a fibrous stroma rich in fibroblasts. Also present were isolated spicules of lamellar bone with osteoblastic rimming of the trabeculae scattered throughout the connective tissue stroma. The pathologic diagnosis was consistent with an ossifying fibroma. Results of the patient’s postoperative evaluation were clinically unremarkable. On radiologic evaluation, the patient had a lesion involving the right sinonasal cavity. This finding suggested residual recurrent tumor at first-year follow-up.
Discussion
Ossifying fibroma is presumed to originate from mesenchymal blast cells. It is a rare, expansile, benign tumor that predominantly involves the maxillary (approximately 10–20% of cases) and mandibular (approximately 75%) bone (8). In rare cases, the tumor may involve the nasal cavity and long bones (8). Patients with ossifying fibroma usually present between the second and fourth decades of life, and female incidence is more frequent than male incidence.
Presenting signs and symptoms are related to the anatomic location of the lesion. For lesions showing benign behavior and not producing deformity, curettage and ostectomy appear adequate (9). After these procedures, the recurrence rate varies from 0% to 28%. If recurrence is detected after curettage, conservative excision is recommended. Aggressive lesions may require en bloc resection (9). Recurrence must be kept in mind when complete resection is not possible because of cosmetic reasons (8), as the lesion is located in the sinonasal region.
Inflammatory and neoplastic lesions of this region must be considered in the differential diagnosis. Inflammatory disease usually has high signal intensity on T2-weighted images and low signal intensity on T1-weighted images as a result of the water component of the sinonasal secretions. When the protein component of the inflammatory lesion increases, its signal intensity increases on T1-weighted images, whereas it progressively declines on T2-weighted images. When the protein component of the inflammatory lesion is higher than 25%, the signal intensity decreases on both T1- and T2-weighted images. The change causes a signal-void pattern resembling that of a normally aerated sinus. This appearance is a major pitfall in the evaluation of chronic sinusitis, sinonasal polyposis, mucoceles, and fungal sinusitis (10).
Mucosal retention cysts arise from inflammatory obstruction of the seromucinous glands. The cysts are seen as smooth-walled, dome-shaped structures. A retention cyst cannot be differentiated from a polyp by means of imaging. Sinus polyps are mainly associated with allergy, inflammation, infection, and cystic fibrosis. The imaging appearance of sinonasal polyposis may mimic that of a malignancy, as it usually fills and expands nasal cavity. Polyposis also causes bone erosion. CT images of polyposis help in differentiating the lesion from a malignancy. In polyposis, CT images show hyperattenuating material in the center, with a peripheral rim of low attenuation, which is characteristic of inflammatory lesions. With its destructive pattern and variable signal-intensity and contrast-enhancement patterns on MR images, fungal sinusitis also leads to problems in the differential diagnosis. Fungal sinusitis may also have findings similar to those of polyposis on CT images. However, the presence of a hyperattenuating focus in the center of an affected sinus that looks like a central, attenuating cast surrounded by a peripheral ring of low attenuation strongly suggests fungal disease (10).
Mucoceles are expansile lesions of sinuses secondary to ostial obstruction. CT reveals a low-attenuating, nonenhancing, expansile lesion. On MR images, the lesion has variable signal intensity. Contrast-enhanced MR images are valuable in differentiating this lesion from neoplasms, as mucoceles show peripheral rim enhancement, whereas sinonasal neoplasms show more-diffuse enhancement (10).
Hence, we can consider CT as an important imaging technique in the differential diagnosis of sinonasal inflammatory lesions from malignancies of this region. MR imaging is useful in the evaluation of the extent and internal component of the lesion. Contrast-enhanced MR images are useful in the differential diagnosis of mucoceles from neoplasms. MR imaging is also useful in the evaluation of any intracranial complications of sinusitis.
Inverted papillomas constitute 75% of all sinonasal papillomas. They are more common in male patients than in female patients, and they mostly arise from the lateral nasal wall. Although these lesions are histologically benign, they are locally aggressive and invade the paranasal sinuses, nasopharynx, and orbit. Inverted papillomas are usually multicentric and associated with a risk of coexisting carcinoma (10).
Adenomas may also arise in the nose and nasopharynx. These masses may simulate nasal polyps, but they are locally invasive and have a risk of recurrence. Pleomorphic adenomas can arise from ectopic glands of the nares. CT and MR images show expansile, destructive lesions without any specific feature that differentiates them from other sinonasal neoplasms (10).
Malignant tumors of sinonasal region are rare, accounting for 3% of all head and neck tumors (10). Squamous cell carcinoma, which accounts for 80% of all sinus malignancies, should also be considered in the differential diagnosis. Undifferentiated sinonasal carcinomas are ill-defined tumors arising from the ethmoid sinus and superior nasal cavity. These lesions are aggressive, with frequent invasion of adjacent structures. These tumors are isointense on T1-weighted images and isointense to hyperintense on T2-weighted images, with heterogeneous enhancement on contrast-enhanced images (10).
Melanomas are rare but possible tumors in the sinonasal region. They have a characteristic imaging appearance, being hyperintense on T1-weighted images and hypointense on T2-weighted images, depending on melanin component of the lesion (10). Lymphoma, plasmocytoma, and metastasis should also be considered in the differential diagnosis of sinonasal lesions (10).
In addition, capillary hemangiomas of the nasal vault should be considered in the differential diagnosis. Patients with these tumors usually present with a history of epistaxis. The lesions typically have intermediate signal intensity on T1-weighted images. They have varying degrees of T2 shortening on T2-weighted images, with an appearance suggesting the presence of blood products. Intense enhancement of the lesion on CT and MR images is a helpful finding in the differentiation of these lesions from others (11)
The main difficulty in differential diagnosis of ossifying fibroma is fibrous dysplasia, because ossifying fibroma may have pathologic areas resembling fibrous dysplasia. Hence, an adequate biopsy sample is needed for differentiation. According to previous studies, ossifying fibroma has sharply circumscribed margins with defined borders merging with normal bone, whereas fibrous dysplasia has diffuse blending and poorly defined margins on radiologic examination. On histopathologic evaluation, ossifying fibroma has lamellar mature bone and closely packed spindle cells that form whorls. In fibrous dysplasia, the stroma is more collagenized and less cellular, with abortive bony trabeculae and woven trabecular bone. A generally uniform bone-to-fibrous tissue ratio with fairly evenly scattered bone trabeculae suggests fibrous dysplasia. Fibrous dysplasia has irregular, haphazardly configured bone trabeculae that are C shaped or fish shaped. However, similar bone trabeculae may be found in ossifying fibroma as well (12, 13).
On radiographs, ossifying fibroma has a somewhat radiopaque, unilocular appearance with distinct boundaries with an eggshell character, whereas the border of fibrous dysplasia is diffuse and merges with normal bone. This finding is useful in differentiating ossifying fibroma from fibrous dysplasia (12, 13). CT examination of an ossifying fibroma usually reveals an eggshell-thin rim of bone surrounding a lytic area (12). CT is also better than radiography in providing valuable information about the extent of the lesion and its relation with surrounding structures. The attenuation of the lesion is usually lower than that of neighboring soft tissues, and older lesions may have striations of calcification (13).
MR imaging of the lesion provides multiplanar information about the extent and characteristics of the lesion. Fibroosseous lesions usually have low to intermediate signal intensity on T1-weighted images and variable signal intensity on T2-weighted images, with some ossified areas having low signal intensity and some areas (central nonossified regions, cysts, or associated mucoceles) having high signal intensity. After the administration of contrast agent, the thick outer layer of the lesion enhances intensely (14, 15). In the case of Engelbrecht et al (15), a thin outer shell with incomplete enhancement was reported.
Fibrous dysplasia is low in signal intensity on T2-weighted images, whereas ossifying fibroma may have high signal intensity, as seen in our case. After the administration of contrast material, fibrous dysplasia has enhancement in only the expanded diploË of the involved bone, whereas in ossifying fibroma, enhancement of the outer shell and septa is observed, as in our case (15). These findings are helpful diagnostic clues in the differentiation of ossifying fibroma from fibrous dysplasia on MR images.
Ossifying fibroma and fibrous dysplasia may share similar pathologic features. Hence, the radiologic features of the lesion should also be considered in the differential diagnosis when pathologic differentiation is uncertain (9).
The presence of a fluid-fluid level on CT or MR images has been reported in many bone and soft-tissue lesions. Fluid-fluid levels may occur whenever substances with different densities are confined to a cystic or compartmental area. This sign is mainly associated with aneurysmal bone cysts and strongly suggestive of them. However, fluid-fluid levels have been reported in association with telangiectatic osteosarcomas, chondrosarcomas, giant cell tumors, osteomyelitis, intraosseous ganglia, simple bone cysts, bone abscesses, metastases, tumoral calcinosis, and cavernous hemangiomas (5, 6). Fluid-fluid levels were also described in a case of fibrous dysplasia with cystic degeneration (6). In addition, fluid-fluid levels in ossifying fibroma have been reported in a pediatric case of ossifying fibroma that was initially diagnosed as an ethmoid mucocele (7). In our case, T2-weighted images of the lesion also showed a fluid-fluid level.
Conclusion
Ossifying fibroma is an extremely rare tumor that may appear with fluid-fluid levels on MR images. Knowledge of its possible locations and characteristic imaging features help in the differential diagnosis of this lesion from other lesions, especially fibrous dysplasia.
References
- 1.Montgomery AH. Ossifying fibroma of the jaws. Arch Surg 1927;15:30 [Google Scholar]
- 2.Tobey JD, Loevner LA, Yousem DM, Lanza DC. Tension pneumocephalus: a complication of invasive ossifying fibroma of the paranasal sinuses. AJR Am J Roentgenol 1996;166:711–713 [DOI] [PubMed] [Google Scholar]
- 3.Marvel JB, Marsh MA, Catlin FI. Ossifying fibroma of the mid-face and paranasal sinuses: diagnostic and therapeutic considerations. Otolaryngol Head Neck Surg 1991;104:803–808 [DOI] [PubMed] [Google Scholar]
- 4.Margo CE, Weiss A, Habal MB. Psammomatoid ossifying fibroma. Arch Ophthalmol 1986;104:1347–1351 [DOI] [PubMed] [Google Scholar]
- 5.Grey AC, Mangham DC, Davies AM, Grimer RJ. Fluid-fluid level in an intraosseous ganglion. Skeletal Radiol 1997;26:667–670 [DOI] [PubMed] [Google Scholar]
- 6.Tsai JC, Dalinka MK, Fallon MD, Zlatkin MB, Kressel HY. Fluid-fluid level: a nonspecific finding in tumors of bone and soft tissue. Radiology 1990;175:779–782 [DOI] [PubMed] [Google Scholar]
- 7.Vaidya AM, Chow JM, Goldberg K, Stankiewicz JA. Juvenile aggressive ossifying fibroma presenting as an ethmoid mucocele. Otolaryngol Head Neck Surg 1998;119:665–668 [DOI] [PubMed] [Google Scholar]
- 8.Choi YC, Jeon E, Park YS. Ossifying fibroma arising in the ethmoid sinus and nasal cavity. Int J Pediatr Otorhinolaryngol 2000;54:159–162 [DOI] [PubMed] [Google Scholar]
- 9.Chong VFH, Tan LHC. Maxillary sinus ossifying fibroma. Am J Otolaryngol 1997;18:419–424 [DOI] [PubMed] [Google Scholar]
- 10.Rao VM, El-Noueam KI. Sinonasal imaging Radiol Clin North Am 1998;6:921–939 [DOI] [PubMed] [Google Scholar]
- 11.Dillon WP, Som PM, Rosenau W. Hemangioma of the nasal vault: MR and CT features. Radiology 1991;180:761–765 [DOI] [PubMed] [Google Scholar]
- 12.Morris MR, Blakeslee DB, Zajtchuk JT. Aggressive paranasal sinus ossifying fibroma. Ear Nose Throat J 1989;68:260–264 [PubMed] [Google Scholar]
- 13.Çakir B, Karaday N. Ossifying fibroma in the nasopharynx. Clin Imaging 1991;15:290–292 [DOI] [PubMed] [Google Scholar]
- 14.Sterling KM, Stollman A, Sacher M, Som PM. Ossifying fibroma of sphenoid bone with coexistent mucocele: CT and MRI. J Comput Assist Tomogr 1993;17:492–494 [DOI] [PubMed] [Google Scholar]
- 15.Engelbrecht V, Preis S, Hassler W, Lenard HG. CT and MRI of congenital sinonasal ossifying fibroma. Neuroradiology 1999;526–529 [DOI] [PubMed]