Abstract
BACKGROUND AND PURPOSE: In the brain of HIV-infected patients, proton MR spectroscopic studies are typically used to examine small volumes of tissue with single-voxel methods. Since brain disease is diffuse in patients with HIV, such studies preclude assessment of the true extent of the metabolic burden. To assess this extent, the relationship between global neuronal integrity, reflected by the whole-brain N-acetylaspartate (WBNAA) concentration, was correlated with neuropsychological function and the AIDS dementia complex (ADC) stage score.
METHODS: WBNAA levels were compared between 15 HIV-infected patients (seven symptomatic, eight asymptomatic) and 13 age- and sex-matched healthy subjects. The patients’ WBNAA level was correlated with cognitive performance, as measured with a battery of eight tests (NPZ-8), including the ADC stage score and four total-memory, mood, motor, and processing speed subtests.
RESULTS: WBNAA levels were significantly different between patients and healthy subjects (mean ± σ, 11.82 ± 1.40 and 12.91 ± 1.03 mmol/L, respectively; P = .032) after we adjusted for age and sex effects. Intermediate negative correlations were found between the WBNAA level, the processing speed subtest score (r = −0.50, P = .03), and the ADC stage score (r = −0.44, P = .05).
CONCLUSION: The WBNAA concentration complements brain atrophy data with information about the quality of the remaining neuronal and axonal tissue in patients with HIV infection. In HIV-infected patients, its correlation with processing speed and the ADC score indicates that the latter reflects pathologic deficits, which are extensive throughout the brain.
AIDS dementia complex (ADC) is a late stage of HIV infection. ADC is a chronic neurodegenerative syndrome characterized by progressive cognitive impairment and brain atrophy and affects 15–20% of patients with AIDS (1). Its diagnosis requires clinical indications of CNS impairment, such as subcortical features of inattention, indifference, and psychomotor slowing before the development of frank dementia (1), in addition to objective measures of dysfunction on standardized neuropsychological tests, such as the NPZ-8 battery (2).
Atrophy is the most common neuroradiographic brain abnormality of ADC, followed by white matter lesions (3). Both are consistent with neuropathologic depictions of diffuse cerebral white matter damage, which is also present in HIV-infected patients without dementia, although to a lesser extent (4, 5). Diffusion tensor imaging similarly demonstrates abnormalities in mean diffusivity and fractional anisotropy in the subcortical white matter of patients with HIV infection, despite the presence of normal-appearing white matter at MR imaging (6, 7).
These findings are consistent with those on localized proton (1H) MR spectroscopic studies of the brain in HIV-infected patients, which show decreased levels of N-acetylaspartate (NAA), a metabolite almost exclusive to neurons and their axonal and dendritic extensions (8), and increased levels of choline (Cho) and myo-inositol (mI). Several groups also report lower NAA/creatine (Cr), (9–17), higher Cho/Cr (10, 13–15, 17, 18), and higher mI/Cr values in such patients (9, 14). Ernst et al (19), however, report significantly lower Cr levels in patients with cognitive and motor dysfunctions, making interpretation of metabolite ratios less definitive. Furthermore, although the effects of HIV in the brain are diffuse, most investigators have used single-voxel methods to examine only 3.5–8-cm3 volumes of tissue, precluding assessment of the true global metabolic load of the disease.
The diffuse extent of HIV in the CNS was recently demonstrated by a correlation between the neuropsychological deficits in patients and their lower percentage of parenchymal brain volume (PBV), an indicator of global atrophy (20). This observation motivated our assessment of a complementary metric—the total neuronal cell injury in the remaining tissue, as reflected by the whole-brain NAA (WBNAA) concentration—in the same patients (21). Our aim was to evaluate the global extent of that damage and its impact on clinical manifestations and cognitive functions.
Methods
Human Subjects
Fifteen patients seropositive for HIV-1 were enrolled. Seven were neurologically symptomatic and eight, asymptomatic. Thirteen age- and sex-matched healthy subjects were also enrolled. Patients were relatively early in the course of their disease, as reflected by ADC scores of 0–1.0. According to this scale, 1 is defined as mild, 0.5 indicates neurologic and neurocognitive impairments but with function in terms of daily living activities, and 0 means asymptomatic. Mean ages were 45 years (range, 40–56 years) for symptomatic patients, 34 years (range, 25–39 years) for asymptomatic patients, and 38 years (range, 27–65 years) for healthy subjects. Patients were prescreened for risk factors for cognitive impairment or evidence of active drug or alcohol abuse. All were deemed clinically stable, without progressive weight loss or constitutional symptoms. None was receiving or ever received long-term corticosteroids, and all but one (patient 10 in the Table ) underwent highly active antiretroviral therapy. Other relevant statistics are compiled in the Table. All the participants provided informed written consent, and our institutional review board approved this study.
Patient Data
| Patient No./Age, y/Sex | Brain Volume, cm3 | PBV | WBNAA, mM | Score |
|||||
|---|---|---|---|---|---|---|---|---|---|
| NPZ-8 | ADC | Total Memory | Motor Speed | Processing Speed | Total Mood | ||||
| 1/37/M | 1264 | 0.83 | 10.1 | 0 | 0 | 44 | 0 | 0 | 215 |
| 2/39/M | 1212 | 0.79 | 11.1 | 0 | 0 | 54 | 0 | 3 | 280 |
| 3/25/M | 1229 | 0.89 | 11.3 | 3 | 0 | 33 | 0 | 0 | 288 |
| 4/40/M | 1180 | 0.83 | 11.4 | 3 | 0 | 31 | 0 | 4 | 282 |
| 5/28/F | 1009 | 0.88 | 13.3 | 7 | 0 | ND | ND | ND | ND |
| 6/30/F | 1119 | 0.92 | 13.3 | 0 | 0 | 49 | 1 | 1 | 264 |
| 7/38/M | 1342 | 0.89 | 13.9 | 0 | 0 | 25 | 0 | 0 | 283 |
| 8/33/M | 1294 | 0.80 | 14.1 | 0 | 0 | 63 | 0 | 0 | 240 |
| 9/45/M | 1252 | 0.78 | 11.1 | 5 | 0.5 | 31 | 2 | 4 | 307 |
| 10/40/F | 974 | 0.79 | 11.7 | 12 | 0.5 | 37 | 6 | 9 | 306 |
| 11/43/M | 830 | 0.65 | 12.1 | 3 | 0.5 | 31 | 4 | 0 | 271 |
| 12/44/M | 1095 | 0.80 | 12.2 | 3 | 0.5 | 39 | 2 | 0 | 306 |
| 13/56/M | 1050 | 0.74 | 12.2 | 5 | 0.5 | 41 | 5 | 3 | 296 |
| 14/45/M | 1002 | 0.73 | 9.7 | 8 | 1 | 31 | 5 | 8 | 259 |
| 15/45/M | 1129 | 0.81 | 9.9 | 6 | 1 | 32 | 1 | 7 | 344 |
Note.—ND indicates no data.
Neuropsychological Evaluation
A standard battery of eight tests, including the California Computerized Assessment Package (Cal CAP) reaction-time tests, was used to derive a composite measure of neuropsychological impairment (NPZ-8 score) (2, 22). These scores ranged from 0 to 15, with higher scores indicating increased neuropsychological dysfunction. ADC staging was determined according to standard clinical criteria described by the American Academy of Neurology (23) and by impaired performance on the NPZ-8 battery, which was defined as a score 2 SD below the mean on one test or a score 1 SD below the mean on two or more tests. Symptomatic was used to describe patients with an ADC stage score ≥0.5, and asymptomatic was used for those whose score was 0 (1). Although we used the term neurologically symptomatic to include any ADC score higher than 0, some investigators designate patients with an apparent ADC score of 0.5 as having only a minor cognitive motor disorder.
To increase the sensitivity of the NPZ-8 and ADC stage tests, we added conceptually derived, composite scores for the following: 1) total memory, which was the sum of the raw scores from the five learning trials of the auditory verbal learning test; 2) motor speed, which was the sum of the age- and education-corrected z scores for Timed Gate and Grooved Pegboard Dominant and non-Dominant hands; 3) processing speed, which was the sum of the age- and education-corrected z scores for Trailmaking Parts A and B, the Symbol Digit Modalities Test, and CalCAP Computerized Reaction Times; and 4) total mood, which was the sum of the t scores for the six scales on the Profile of Mood States. (See the article by Patel et al for details [20]). The NPZ-8 composite, ADC stage, and four subtest scores were the functional parameters; we investigated the relationship of these parameters to WBNAA levels.
WBNAA Quantification
The amount of WBNAA, QNAA, was obtained by using a nonlocalizing proton MR spectroscopic sequence described previously (21). The protocol, including subject placement, shimming, and MR spectroscopy, required less than 25 minutes to complete. Absolute quantification of the WBNAA signal intensity was performed against a reference 3-L sphere containing 1.5 × 10−2 mol NAA in water. Subject and reference NAA peak areas, SS and SR, respectively, were integrated. The subject’s QNAA was calculated by using Equation 1 (21):
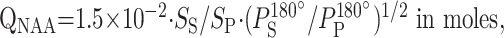 |
1) |
where PR180° and PS180° are the transmitter power needed for a nonselective, 1-ms, 180° inversion pulse on the phantom and subject, respectively. These values reflect the sensitivity of the system. QNAA represents >90% of NAA in the brain, missing only regions of severe air-tissue susceptibility differences (eg, in the mesial temporal and lower frontal lobes and near the auditory canals) (24). Because this inversion recovery is tuned to the T1 of NAA and because it relies on the prior knowledge that the latter is found only in neuronal cells for localization, it neither correctly quantifies nor localizes the other metabolites (eg, Cho, Cr, mI), which are also present in head muscle and lipid tissue. Consequently, only quantitative NAA values are referenced in the ensuing discussion.
To normalize the values for intersubject variations in brain size, QNAA was divided by that individual’s brain volume, VB, obtained from high-resolution MR images (256 × 256 matrix, 220 × 220-mm2 field of view, 3-mm section thickness, dual fast spin-echo sequence with TE1 and TE2/TR = 16 and 80/2500, respectively) obtained within 2 days of neuropsychological testing. The images were segmented into CSF and parenchyma. The volumes of the former, PBV, and the latter, VB, are given in the Table. These volumes were the sums of the pixels in each moiety. Finally, the WBNAA concentration and PBV were defined in Equations 2 and 3: (21),
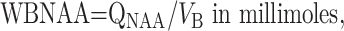 |
2) |
and
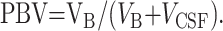 |
3) |
Because neither metric depends on brain size, both are suitable for cross-sectional comparisons.
Statistical Analysis
Least-squares regression was used to compare the WBNAA levels in patients with those of healthy subjects after we adjusted for age- and sex-attributable differences. The regression model used to predict WBNAA values was linear for age and included sex and the disease state as fixed classification factors. The bivariate associations between WBNAA values and all of the other metrics referenced in the Table were assessed by using Spearman rank correlation coefficients.
Results
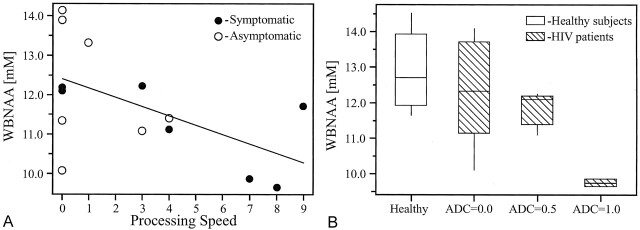
WBNAA levels were significantly different between HIV-infected patients and healthy subjects (mean ± σ, 11.82 ± 1.40 and 12.91 ± 1.03 mmol/L, respectively; P = .032) after we adjusted for age and sex effects. When the WBNAA levels were compared between healthy subjects and asymptomatic patients (12.31 ± 1.51 mmol/L) or symptomatic patients (11.27 ± 1.10 mmol/L), only the latter values were significantly different from those of the healthy subjects (P = .008). Spearman rank correlation analysis of the 15 HIV-infected patients revealed a significant intermediate negative correlation (r = −0.499; P = .035) between subtest scores for processing speed and WBNAA level (Fig 1A). Because of the granularity of the scale in this cohort (only three discreet values, as shown in Fig 1B), Spearman rank correlation coefficient analysis was also used. The results showed a significant negative correlation between the ADC stage score and the WBNAA value (r = −0.438, P = .05). No significant correlations were found between WBNAA and any of the other metrics in the Table, ie, total memory impairment, motor speed impairment, total mood dysfunction, composite NPZ-8 score, or PBV.
Fig 1.
Analysis of WBNAA concentration versus processing speed or ADC score.
A, WBNAA concentrations as a function of processing speed for each of the 15 HIV-infected patients. The solid line is the least-squares regression model used to predict WBNAA concentration (in millimoles) from the processing speed in HIV-infected patients, as follows: WBNAA = 12.38 − 0.2357 × processing speed. A higher processing speed score indicates increased impairment.
B, Box plots display the 25% (box bottom), median, 75% (box top) values and the ±95% (whiskers) range of the variation in WBNAA concentrations for HIV-infected patients in each of the ADC score categories and for their age- and sex-matched healthy subjects.
Discussion
Although successful antiretroviral therapy has led to a downward trend in the incidence of ADC, dementia still develops in about 15–20% (60,000–150,000) of all AIDS patients in the United States (1). The risk for this progression motivates the search for and the development of neuroimaging markers that can be used to identify and quantify HIV damage to the CNS in the earlier stages of the disease and to establish their connection with common clinical metrics. Among the markers frequently examined are global atrophy and lesion load, obtained from MR image segmentation and local metabolic characteristics from proton MR spectroscopy (10, 13–15, 17, 18).
Indeed, our previous study in a cohort of HIV-infected patients showed that progressive brain atrophy is correlated with neurocognitive deficits. The findings suggested that greater loss of brain parenchyma may be predictive of poorer neuropsychological and clinical performance (20). While whole-brain volumetry is a reliable marker of the sum of all the pathologic processes, it also has several intrinsic limitations. Specifically, 1) it reflects not only neuronal and axonal losses but also a reduction of axonal diameter and myelin loss, 2) it can be partially masked (offset) by reactive gliosis, and 3) it yields no information about the quality of the remaining tissue.
In contrast to brain volumetry, the NAA concentration provides specific information about the health and density of the remaining amount of neurons and axons. To quantify that metric, we used WBNAA studies instead of one of the localized proton MR spectroscopic techniques used to examine small (approximately 3 to <cm3) regions, especially because HIV diffusely affects the entire brain (9). The tradeoff for this extensive (>90%) brain coverage (24) is a sensitivity to (restricted) regional disease that is lower than that of single-voxel proton MR spectroscopy. That higher sensitivity, however, is realized only 1) if the voxel is assumed to be placed right on a pathologic locus or 2) if it is assumed that the disease is homogeneous (ie, all locations are equivalent). Actual diffuse diseases, however, have no single unique locus to probe, and although all (or at least most) of the CNS is involved, various regions may be affected differently. In contrast, WBNAA requires neither assumption.
Therefore, the moderate correlations (0.4 ≤ r ≤ 0.7) found between the clinical and neurocognitive deficits reflected by the ADC stage and between processing speed scores and WBNAA value confirm that diffuse neurodegeneration occurs, even when severe clinical dysfunction is absent. Indeed, in postmortem studies, HIV-infected patients with cognitive and motor impairment were found to have neuronal loss in the cortex and axonal loss in the white matter of 18–50% (1). This observation substantiates our noninvasive results in living subjects. Although WBNAA values were not useful for distinguishing patients with an ADC score of 0 from control subjects (Fig 1B), this finding is perhaps unsurprising, given the trade-off between sensitivity and greater spatial coverage with the WBNAA method.
Interestingly, while PBV was previously found to be correlated with the subtest score for motor impairment but not with processing speed (20), WBNAA values showed an inverse trend in the same cohort, being correlated with the latter parameter but not the former. We believe that the limited spatial extent of neuronal loss (mainly in the basal ganglia and frontal cortex) in HIV infection may be too small to significantly affect the global neuronal and axonal pools. Conversely, the processing speed score, which includes attention, concentration, and memory functions, involves enough white matter pathways and connecting cortical and subcortical structures to be detectable with this method. Additionally, while WBNAA concentration is a more specific metric for tissue damage than atrophy is, it cannot be used to distinguish distributed, local foci of neuronal or axonal loss from more extensive (but less severe) dysfunction (ie, total versus partial, perhaps even reversible, NAA loss). Because only one time point was examined, this issue of permanent versus partial and transient loss could not be addressed in this study.
Since the WBNAA level reflects both focal and widespread diffuse damage, it is a better potential correlate with neurologic functions controlled by (more spatially expansive) multicircuit networks (eg, cognition) than those governed by well-defined brain regions (eg, motility, sensation, balance). Although adaptive cortical reorganization (plasticity) could lead to an underestimation of the true extent of the damage with neuropsychological examinations, WBNAA values (which are used to monitor metabolism and not function) is immune to this confounding factor. In this context, the WBNAA concentration may be a better tool than localized metrics, such as single-voxel proton MR spectroscopic findings or even specialized (and therefore spatially localized) clinical and performance outcomes, in quantifying the true extent of the underlying brain injury, which may be present despite seemingly preserved clinical performance.
Conclusion
Brain atrophy and WBNAA values (as determined with MR imaging and proton MR spectroscopy) provide complementary data about the amount of parenchyma lost and about the quality of the remaining neuronal and axonal tissue, respectively, in the entire brain of patients with HIV infection. Since both measures are correlated with (different) cognitive parameters, they could potentially provide objective (ie, instrumental) metrics in this patient population, which is frequently ill suited for the former (subjective) assessment. Furthermore, because the whole brain is assessed, global diffuse damage can be detected, even if specific circuits examined by using clinical tests are (still) spared.
Footnotes
This work was supported by National Institutes of Health grants EB01015, NS37739, CA92547, and NS29029.
References
- 1.McArthur JC, Sacktor N, Selnes O. Human immunodeficiency virus-associated dementia. Semin Neurol 1999;19:129–150 [DOI] [PubMed] [Google Scholar]
- 2.Navia BA, Dafni U, Simpson D, et al. A phase I/II trial of nimodipine for HIV-related neurologic complications. Neurology 1998;51:221–228 [DOI] [PubMed] [Google Scholar]
- 3.Bencherif B, Rottenberg DA. Neuroimaging of the AIDS dementia complex. Aids 1998;12:233–244 [PubMed] [Google Scholar]
- 4.Power C, Kong PA, Crawford TO, et al. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier. Ann Neurol 1993;34:339–350 [DOI] [PubMed] [Google Scholar]
- 5.Gray F, Scaravilli F, Everall I, et al. Neuropathology of early HIV-1 infection. Brain Pathol 1996;6:1–15 [DOI] [PubMed] [Google Scholar]
- 6.Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in HIV-1 infection: a diffusion tensor imaging study. Psychiatry Res 2001;106:15–24 [DOI] [PubMed] [Google Scholar]
- 7.Filippi CG, Ulug AM, Ryan E, Ferrando SJ, van Gorp W. Diffusion tensor imaging of patients with HIV and normal-appearing white matter on MR images of the brain AJNR Am J Neuroradiol 2001;22:277–283 [PMC free article] [PubMed] [Google Scholar]
- 8.Simmons ML, Frondoza CG, Coyle JT. Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience 1991;45:37–45 [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Villegas D, Lenkinski RE, Frank I. Biochemical changes in the frontal lobe of HIV-infected individuals detected by magnetic resonance spectroscopy. Proc Natl Acad Sci U S A 1997;94:9854–9859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moller HE, Vermathen P, Lentschig MG, et al. Metabolic characterization of AIDS dementia complex by spectroscopic imaging. J Magn Reson Imaging 1999;9:10–18 [DOI] [PubMed] [Google Scholar]
- 11.Wilkinson ID, Miller RF, Miszkiel KA, et al. Cerebral proton magnetic resonance spectroscopy in asymptomatic HIV infection. Aids 1997;11:289–295 [DOI] [PubMed] [Google Scholar]
- 12.Meyerhoff DJ, MacKay S, Bachman L, et al. Reduced brain N-acetylaspartate suggests neuronal loss in cognitively impaired human immunodeficiency virus-seropositive individuals: in vivo 1H magnetic resonance spectroscopic imaging. Neurology 1993;43:509–515 [DOI] [PubMed] [Google Scholar]
- 13.Chong WK, Sweeney B, Wilkinson ID, et al. Proton spectroscopy of the brain in HIV infection: correlation with clinical, immunologic, and MR imaging findings. Radiology 1993;188:119–124 [DOI] [PubMed] [Google Scholar]
- 14.Laubenberger J, Haussinger D, Bayer S, et al. HIV-related metabolic abnormalities in the brain: depiction with proton MR spectroscopy with short echo times. Radiology 1996;199:805–810 [DOI] [PubMed] [Google Scholar]
- 15.Jarvik JG, Lenkinski RE, Grossman RI, Gomori JM, Schnall MD, Frank I. Proton MR spectroscopy of HIV-infected patients: characterization of abnormalities with imaging and clinical correlation. Radiology 1993;186:739–744 [DOI] [PubMed] [Google Scholar]
- 16.McConnell JR, Swindells S, Ong CS, et al. Prospective utility of cerebral proton magnetic resonance spectroscopy in monitoring HIV infection and its associated neurological impairment. AIDS Res Hum Retrovir 1994;10:977–982 [DOI] [PubMed] [Google Scholar]
- 17.Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology 1999;52:100–108 [DOI] [PubMed] [Google Scholar]
- 18.Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, Weiner MW, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology 1999;52:995–1003 [DOI] [PubMed] [Google Scholar]
- 19.Ernst T, Itti E, Itti L, Chang L. Changes in cerebral metabolism are detected prior to perfusion changes in early HIV-CMC: a coregistered (1)H MRS and SPECT study. J Magn Reson Imaging 2000;12:859–865 [DOI] [PubMed] [Google Scholar]
- 20.Patel SH, Kolson DL, Glosser G, et al. Correlation between percentage of brain parenchymal volume and neurocognitive performance in HIV-infected patients. AJNR Am J Neuroradiol 2002;23:543–549 [PMC free article] [PubMed] [Google Scholar]
- 21.Gonen O, Viswanathan AK, Catalaa I, Babb J, Udupa J, Grossman RI. Total brain N-acetylaspartate concentration in normal, age- grouped females: quantitation with non-echo proton NMR spectroscopy. Magn Reson Med 1998;40:684–689 [DOI] [PubMed] [Google Scholar]
- 22.Dana T. Consortium on Therapy for HIV Dementia and Related Cognitive Disorders: a randomized, double-blind, placebo-controlled trial of deprenyl and thioctic acid in human immunodeficiency virus-associated cognitive impairment—Dana Consortium on the Therapy of HIV Dementia and Related Cognitive Disorders. Neurology 1998;50:645–651 [DOI] [PubMed] [Google Scholar]
- 23.Clinical confirmation of the American Academy of Neurology algorithm for HIV-1-associated cognitive/motor disorder—The Dana Consortium on Therapy for HIV Dementia and Related Cognitive Disorders. Neurology 1996;47:1247–1253 [DOI] [PubMed] [Google Scholar]
- 24.Gonen O, Grossman RI. The accuracy of whole brain N-acetylaspartate quantification. Magn Reson Imaging 2000;18:1255–1258 [DOI] [PubMed] [Google Scholar]