Abstract
BACKGROUND PURPOSE: Endovascular aneurysm therapy has associated risks of ischemic complications. We undertook this study to evaluate the efficacy of neurophysiological monitoring (NPM) techniques in the detection of ischemic changes that may be seen during endovascular treatment of cerebral aneurysms.
METHODS: Thirty-five patients underwent NPM during endovascular treatment of cerebral aneurysms. The patients underwent a total of 50 endovascular procedures, including balloon test occlusion (19 patients), GDC embolization (22 patients), and permanent vessel occlusion (nine patients). NPM included electroencephalography, somatosensory evoked potentials, and/or brain stem auditory evoked potentials, depending on the location of the aneurysm.
RESULTS: NPM changes were seen in nine (26%) of 35 patients and altered the management in five (14%) of 35 patients. In three of the five cases, NPM changes were observed without corresponding neurologic physical examination changes after balloon test occlusion (performed while the patients were under general anesthesia in two cases). In the two other cases in which NPM changes altered management, ischemia was detected at the time of intra-aneurysmal therapy while the patients were under general anesthesia. Overall, 18 of 35 patients underwent a total of 19 balloon test occlusion procedures. Of the 17 remaining patients, 13 underwent aneurysm coiling, two were not treated because of inability to safely place coils, and two were treated for distal aneurysms. Two patients developed transient neurologic deficits without concurrent NPM changes, representing false-negative NPM test results.
CONCLUSION: NPM is a valuable adjunct to endovascular treatment of cerebral aneurysms. Our study suggests that these monitoring techniques may reduce ischemic complications and can be used to help guide therapeutic decisions.
Endovascular treatment of aneurysms can alter regional cerebral blood flow (rCBF) in the arterial distribution of the aneurysm and cause attendant ischemic complications. Treatment may require parent vessel occlusion, which directly influences blood flow. In addition, intra-aneurysmal therapy may result in thromboembolic complications or may partially impede blood flow in the parent vessel. These therapeutic maneuvers often are performed with the patients under general anesthesia. Therefore, a technique to detect and monitor significant changes in rCBF at the time of endovascular treatment of aneurysms may improve patient outcomes.
Direct measures of rCBF include radionuclide CBF studies, transcranial Doppler sonography, and xenon CT. Some authors have used a technique such as xenon CT to evaluate CBF flow during balloon test occlusion after transferring patients from the angiography suite to a CT scanner (1). However, these techniques often are difficult to use repeatedly or continuously during endovascular procedures.
Neurophysiological monitoring (NPM) can directly assess the functional state of specific cerebral regions and provides an indirect measure of regional ischemia produced in the cerebral circulation. It can be used at the time of the endovascular aneurysm treatment and provides continuous monitoring of the patient. The relationship of changes in rCBF with changes in NPM has been well established in animal models (2–5) and in the surgical literature (6–8). Hacke et al (9) first reported the use of NPM in interventional neuroradiology. Since then, relatively few reports (10–18) have been published for a variety of applications in interventional neuroradiology, including carotid artery test occlusion, arteriovenous malformation embolization, and basilar artery thrombolysis. The purpose of our study was to assess the efficacy of multimodality NPM in detecting significant ischemia during endovascular treatment of cerebral aneurysms at the time of intra-aneurysmal GDC coil embolization or permanent vessel occlusion.
Methods
The study was a retrospective evaluation of patients treated during a 24-month period ending in June 1999. Thirty-five patients ranging in age from 33 to 81 years (mean age, 59.9 years) who underwent NPM during endovascular treatment of cerebral aneurysms were included. At our institution, personnel for NPM are available for nearly all elective and emergency cases. Many of the patients treated in this study were treated on an emergency basis. At the time of the study, only one physician was actively involved in NPM; currently, two are actively involved. For this reason, two patients who received endovascular therapy during the study period did not undergo NPM because it was unavailable at the time of treatment. They were therefore excluded from the study.
Of the 35 patients included in the study, 33 were deemed to be poor surgical candidates because of age, aneurysm size/morphology, or preexisting medical conditions and two had requested endovascular aneurysm treatment. Eleven of 35 patients had ruptured aneurysms, whereas 24 of 35 had unruptured aneurysms. The demographic characteristics of these patients are summarized in Table 1.
TABLE 1:
Patient demographic data
| Patient No. | Age (yr) | Sex | Aneurysm Location | Ruptured (Y/N) | Procedure | NPM Type |
|---|---|---|---|---|---|---|
| 1 | 80 | F | LICA | N | Aneurysm coiling | SSEP |
| 2 | 33 | F | R cavemous ICA | N | Aneurysm coiling | SSEP |
| 3 | 41 | F | Basilar trunk | N | Aneurysm coiling | SSEP, BAEP |
| 4 | 54 | M | LICA | N | Aneurysm coiling | SSEP, EEG |
| 5 | 40 | F | RICA | N | BTO s/p intracranial bypass | SSEP, EEG, neuro exam |
| BTO and RICA occulusion | SSEP, EEG, neuro exam | |||||
| 6 | 52 | F | RICA ophthalmic | Y | BTO and aneurysm coiling | SSEP, EEG |
| 7 | 59 | M | Distal L PICA | Y | Sodium amytal testing and glue embolization of L PICA | SSEP, BAEP |
| 8 | 48 | F | MCA | Y | BTO and RICA occlusion* | SSEP, BAEP, neuro exam |
| 9 | 52 | F | Basilar tip | Y | Attempted aneurysm coiling | SSEP, BAEP |
| 10 | 81 | F | RICA | N | BTO | SSEP, EEG, neuro exam |
| 11 | 80 | F | Basilar | N | BTO and RVA occlusion | SSEP, BAEP |
| 12 | 74 | F | RICA | N | BTO and aneurysm coiling | SSEP, EEG, neuro exam |
| 13 | 72 | M | R AICA | Y | Aneurysm coiling | SSEP, EEG |
| 14 | 76 | F | R supraclinoid ICA | N | BTO and aneurysm coiling | SSEP, BAEP, neuro exam |
| 15 | 76 | M | Mid-basilar | N | BTO and LVA occlusion† | SSEP, BAEP, neuro exam |
| 16 | 54 | F | R Pcomm | N | Pre-op BTO | EEG, neuro exam |
| 17 | 67 | F | L para-ophthalmic ICA | N | BTO and aneurysm coiling | SSEP, EEG |
| 18 | 52 | F | LICA | N | BTO and aneurysm coiling | SSEP, EEG, neuro exam |
| 19 | 41 | F | L paraclinoid | N | Aneurysm coiling | SSEP, EEG |
| 20 | 52 | M | LICA | N | Aneurysm coiling | SSEP, EEG |
| 21 | 49 | F | L cavernous ICA | N | Pre-op BTO | SSEP, neuro exam |
| LICA occlusion | SSEP, EEG, neuro exam | |||||
| 22 | 72 | F | R cavernous ICA | N | BTO and RICA occlusion | SSEP, EEG |
| 23 | 76 | F | R MCA bifurcation | N | Aneurysm coiling | SSEP, EEG |
| 24 | 43 | M | R distal vertebral | N | BTO and RVA occlusion | SSEP, BAEP, neuro exam |
| 25 | 60 | M | L para-ophthalmic ICA | N | BTO and aneurysm coiling | SSEP, EEG, neuro exam |
| 26 | 78 | F | L MCA bifurcation | Y | Attempted aneurysm coiling | SSEP, EEG |
| 27 | 75 | F | L distal ICA | Y | BTO and attempted aneurysm coiling | SSEP, EEG |
| 28 | 54 | M | Basilar tip | Y | Aneurysm coiling | SSEP, BAEP |
| 29 | 73 | F | Basilar tip, Acomm, R Pcomm | N | Aneurysm coiling | SSEP, BAEP |
| 30 | 40 | F | R MCA bifurcation | Y | Aneurysm coiling | SSEP |
| 31 | 68 | F | Basilar tip | N | Aneurysm coiling | SSEP, BAEP |
| 32 | 62 | F | L paraclinoid ICA | Y | Aneurysm coiling | SSEP, EEG |
| 33 | 44 | M | R P2 segment | N | Sodium amytal testing | SSEP, BAEP, neuro exam |
| Aneurysm coiling | SSEP, BAEP, EEG | |||||
| 34 | 69 | F | LICA | Y | BTO and aneurysm coiling | SSEP, EEG |
| 35 | 51 | M | L distal VA | N | BTO and LVA occlusion | SSEP, BAEP, neuro exam |
Note.—Y indicates yes; N, no; NPM, neurophysiological monitoring; F, female; M, male; L, left; R, right; ICA, internal carotid artery; PICA, posterior inferior cerebellar artery; MCA, middle cerebral artery; AICA, anterior inferior cerebellar artery; Pcomm, posterior communicating artery; Acomm, anterior communicating artery; VA, vertebral artery; BTO, balloon test occlusion; s/p, status-post; pre-op, preoperative; SSEP, somatosensory evoked potential; BAEP, brain stem auditory evoked potential; neuro exam, neurologic examination.
Surgical ligation of the left internal carotid artery.
Basilar artery test occlusion failed for this patient; permanent left vertebral artery occlusion was performed.
All GDC embolization procedures were performed with the patients under general anesthesia to minimize patient motion. Conscious sedation was used whenever possible for patients who required balloon test occlusion of the parent vessel before definitive treatment, to perform neurologic examination testing in addition to NPM. Patients who tolerated the temporary balloon occlusion were often then placed under general anesthesia, for either GDC embolization or permanent vessel occlusion, and underwent continuous NPM. For four patients, conscious sedation was not possible during balloon test occlusion and general anesthesia was used. Therefore, only NPM (without neurologic physical examination) was performed in these cases.
Electrophysiological Monitoring
NPM included EEG, somatosensory evoked potentials (SSEPs), and/or brain stem auditory evoked potentials (BAEPs), based on the location of the aneurysm and vascular territory at risk (Table 1). In cases in which the internal carotid artery and/or middle cerebral artery vascular territory was at risk, NPM was accomplished by using a combination of EEG and SSEP testing (performed after bilateral median nerve stimulation). Anterior cerebral artery territory aneurysm treatment (especially anterior communicating artery) was monitored with SSEP testing by using bilateral median and posterior tibial nerve stimulation. Vertebral and basilar artery vascular supply required a combination of median nerve-generated cortical SSEPs and BAEPs, which were acquired after bilateral independent stimulation.
In elective cases, preoperative EEG, SSEP, and/or BAEP testing was performed in addition to a thorough neurologic examination during a clinic visit. No adverse effects were observed in those patients who underwent preoperative evoked potential testing. In emergency cases, pre-procedural baseline monitoring was performed while the patient was on the angiography table and a limited clinical neurologic examination was also performed of conscious patients.
A Nicolet Viking IV Electrodiagnostic System (Nicolet Instrument Corporation, Madison, WI) was used to obtain the evoked potentials. Standard surface EEG electrodes were used and placed, by using collodion (except for Erb’s point), according to the International 10–20 Electrode Placement System. Recording electrode positions included bilateral Erb’s point, cervical spine (C7), C3′, C4′, CZ, and CZ′ for SSEP recordings, and CZ for BAEP recordings. A frontal (FZ) reference was used for SSEPs, and an ear (A1/A2) was used for BAEPs. For SSEP recordings, bilateral alternating side-to-side stimulation was performed. Constant current stimulation of 0.2-ms duration was delivered via standard surface bar electrodes with a cathode 3 cm proximal to the anode. Stimulation intensity was sufficient to elicit thumb twitch or a clear Erb’s point evoked potential. Rate of stimulation varied between 3.7 and 4.7 Hz. Electrode impedances were kept below 5000 ohms. Filters were routinely set at 30 and 3000 Hz. Sweep time was at 50 ms (median nerve) and 100 ms (posterior tibial nerve). A minimum of 250 responses were averaged.
BAEPs were generated by applying transducer-induced ear clicks by using ear inserts. Click polarity was alternating and was delivered at a rate between 10.7 and 11.1 Hz. One thousand responses were averaged. Click intensity varied from patient to patient but did not exceed 105 dB. White noise of appropriate intensity was always applied to the contralateral ear.
SSEP recordings were analyzed by using both the latency and amplitude (N19-P24) of the cerebral generated evoked responses. Critical SSEP changes were defined as an amplitude reduction of the cerebral evoked potential of >50% or a latency delay (N19/P24) of >10%. BAEP analysis was accomplished by using the latency and amplitude of all peaks (I–V). Changes in BAEPs that were thought to be significant included >50% amplitude reduction of waves III or V and/or an increase in latency of the fifth peak or of the interpeak latency difference (PV − PI) >1 ms.
Temperature and anesthesia effects were taken into consideration for both the SSEPs and the BAEPs. Changes were further classified as permanent if the changes persisted to the end of the procedure and transient if the changes recovered to >50% of baseline before completion of endovascular treatment.
Results
Eighteen patients underwent 19 balloon test occlusion procedures. For each of four patients, a positive (failed) test led to a decision not to perform parent vessel occlusion. Two of the four patients underwent balloon test occlusion while under general anesthesia because of their inability to tolerate conscious sedation (patients 11 and 27), and two underwent the test under conscious sedation (patients 5 and 10). Two of the patients received no further endovascular treatment, one underwent delayed permanent vessel occlusion approximately 6 weeks later (to allow maturation of an extracranial-intracranial bypass graft), and one underwent permanent vessel occlusion of the distal right vertebral artery (rather than the basilar artery). Overall, among the 18 patients tested, eight underwent permanent vessel occlusion (including one patient for whom initial balloon test occlusion had failed but for whom repeat test occlusion after intracranial bypass surgery subsequently passed), seven underwent aneurysm coiling, and three received no further endovascular treatment.
Among the 17 remaining patients, 13 underwent aneurysm coiling, two were not treated because of inability to safely place coils, and two were treated for distal aneurysms (after provocative sodium amytal testing) by glue embolization (1) or permanent vessel occlusion (1).
Table 2 lists the patients for whom NPM or physical examination changes were identified and describes when these changes altered our treatment decisions. NPM changes were observed for nine (26%) of 35 patients and altered management for five (14%) of 35 patients. For three of the five patients, NPM changes were observed without corresponding neurologic physical examination changes after balloon test occlusion (performed with the patient under general anesthesia in two of three cases). For the other two of five patients for whom NPM changes altered management, ischemia was detected at the time of intra-aneurysmal therapy while patients were under general anesthesia.
TABLE 2:
Patients with changes in results of neurophysiological monitoring or NPM or clinical examination during endovascular procedures
| Patient No. | General Anesthesia (Y/N)* | NPM Changes (Y/N) | PE Changes (Y/N) | Altered Management (Y/N) | Type of Alteration/Reason for No Alteration |
|---|---|---|---|---|---|
| 4 | Y | Y | N | N | Coil loop protruding from aneurysm; coil not withdrawn |
| 5 | N | Y | N | Y | Delayed PVO to allow EC-IC graft maturation |
| 7 | Y | Y | N | Y | Increased mean arterial pressure to improve perfusion |
| 9 | Y | Y | N | N | Aneurysm perforation; proceeded with treatment |
| 11 | Y | Y | N | Y | PVO of right vertebral instead of basilar artery |
| 26 | Y | Y | N | Y | No coil detachment |
| 27 | Y | Y | N | Y | No coiling or PVO |
| 29 | Y | Y | N | N | Aneurysm perforation; proceeded with treatment |
| 30 | Y | Y | N | N | NPM changes at end of procedure |
| 10 | N | N | Y | Y | No PVO |
| 33 | N | N | Y | Y | No PVO |
Note.—Y indicates yes; N, no; NPM, neurophysiological monitoring; PE, physical examination; PVO, permanent vessel occlusion; EC-IC, extracranial-intracranial.
Balloon test occlusion was performed with the patient under conscious sedation when possible; permanent vessel occlusion or aneurysm coiling was then performed with the patient under general anesthesia.
NPM changes indicating cerebral ischemia altered treatment decisions for five patients as follows: 1) permanent vessel occlusion was delayed for 6 weeks to allow for maturation of an extracranial-to-intracranial bypass graft (patient 5), 2) permanent vessel occlusion was performed in the right vertebral artery rather than the basilar artery (patient 11), 3) GDCs were not deployed in an aneurysm because of ischemic changes that occurred with coil placement (patient 26) (Fig 1), 4) mean arterial pressure was increased during superselective catheterization to increase cerebral perfusion (patient 7), and 5) permanent vessel occlusion was not performed in wide neck aneurysms (patient 27) (Fig 2). The NPM changes resolved and returned to normal or nearly normal in all five cases, and none of these patients had permanent neurologic deficits.
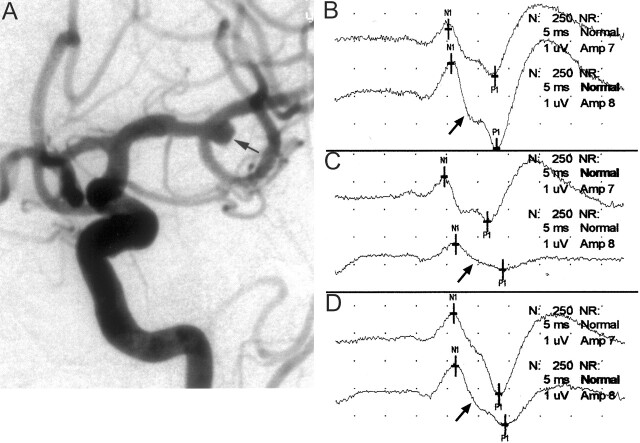
Fig 1.
Images from the case of a 78-year-old woman who presented with symptoms of a subarachnoid hemorrhage.
A, Left internal carotid artery injection in the anterolateral projection shows a 5-mm left middle cerebral artery bifurcation aneurysm (arrow). Intra-aneurysmal coiling was attempted while the patient was systemically heparinized.
B, Baseline cerebral SSEPs after bilateral median nerve stimulation. Top tracing, left median nerve stimulation (ie, right brain); bottom tracing, right median nerve stimulation (ie, left brain) (arrow).
C, One minute after coil placement into the aneurysm, a >50% decrease in amplitude of the right median nerve SSEP was noted (arrow). This is consistent with significant left cerebral ischemia. Fluoroscopic evaluation suggested the coil was partially prolapsed into the parent artery, and considering the change in potentials, it was decided to quickly remove this coil. Formal angiographic assessment may well have shown significant compromise in the parent vessel; however, because the changes were rapid and profound, the coil was removed. Top tracing, left median nerve stimulation (ie, right brain); bottom tracing, right median nerve stimulation (ie, left brain) (arrow).
D, Left cerebral evoked potential (arrow) returned to baseline levels after removal of the coil. Because coil embolization could not be performed safely, the patient subsequently underwent surgical clipping of the aneurysm. Top tracing, left median nerve stimulation (ie, right brain); bottom tracing, right median nerve stimulation (ie, left brain) (arrow).
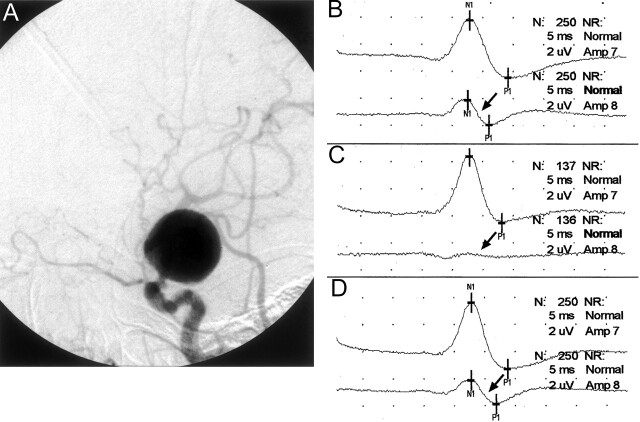
Fig 2.
Images from the case of a 75-year-old woman who presented with a ruptured giant left internal carotid artery aneurysm.
A, Lateral projection angiogram of the left internal carotid artery shows the aneurysm arising in the supraclinoid segment shortly after the takeoff of the ophthalmic artery. The aneurysm was thought to be unfavorable for GDC embolization, and balloon test occlusion was thus performed in anticipation of permanent vessel occlusion. Because of the patient’s physical condition, the procedure could be performed only with the patient under general anesthesia.
B, Baseline cerebral SSEPs after bilateral median nerve stimulation. Note the baseline asymmetry, with the right median nerve SSEP (arrow) being smaller in amplitude than the left. Top tracing, left median nerve stimulation (ie, right brain); bottom tracing, right median nerve stimulation (ie, left brain) (arrow).
C, Left internal carotid artery balloon test occlusion was performed, resulting in gradual amplitude reduction of the left cerebral (right median nerve stimulation) SSEP over 5 min and precipitous decrease in the 6th min. The SSEP obtained 6 min after balloon occlusion shows a nearly complete loss of the left cerebral SSEP (arrow). Top tracing, left median nerve stimulation (ie, right brain); bottom tracing, right median nerve stimulation (ie, left brain) (arrow).
D, Balloon was deflated immediately after the SSEP tracing shown in 2C. The cerebral SSEP returned to baseline amplitude levels after 1 min (arrow). Top tracing, left median nerve stimulation (ie, right brain); bottom tracing, right median nerve stimulation (ie, left brain) (arrow).
Among the four patients for whom NPM changes did not alter treatment decisions, the reasons were as follows: 1) aneurysm rupture (in two cases) required continuation of the procedure despite evidence of cerebral ischemia (patients 9 and 29); 2) a small clot was seen in the M1 segment of the middle cerebral artery during ipsilateral internal carotid artery aneurysm coiling, but thrombolysis was not attempted because of aneurysm perforation that had occurred during aneurysm coiling (patient 4); and 3) diffuse NPM changes due to cerebral vasospasm were identified with successful aneurysm coiling, and the decision was made to treat the vasospasm systemically (patient 30).
None of the patients in this series had both positive NPM and physical examination changes. Two patients did, however, develop transient neurologic deficits without concurrent NPM changes at the time of the procedure: 1) facial droop during balloon test occlusion (patient 10), and 2) new quadrantanopia after sodium amytal in the appropriate vascular territory (patient 33). The transient deficits resolved completely within 10 min. These were classified as false-negative NPM results, even though the affected cortical territory did not correspond to the cerebral distribution tested by the evoked potentials.
Six (11.5%) delayed ischemic complications in patients with no NPM changes occurred after therapy, at 15 hr to 5 days after treatment, among our 52 procedures. In five cases, radiologic follow-up showed these infarcts to be embolic (generally small cortical lesions) and they were therefore not considered false-negative responses to NPM. In one case involving a fusiform aneurysm of the basilar artery, a complication due to hypoperfusion was initially suspected but was also later shown to be embolic in nature: a left posterior inferior cerebellar artery territory infarction occurred after the patient discontinued his regimen of warfarin anticoagulation.
Discussion
To our knowledge, this study represents the first series describing the routine use of NPM during endovascular treatment of cerebral aneurysms. NPM including EEG and evoked potential is particularly valuable when the patient must be placed under general anesthesia for balloon test occlusion or for GDC aneurysm embolization because concurrent neurologic examination is not possible. Alternative approaches to balloon test occlusion have been published (1). These include the use of xenon CT to assess cerebral perfusion after a clinical neurologic examination with the balloon inflated. This has the disadvantage of requiring the patient to be transported to the CT scanner, where the balloon is again inflated in a blinded fashion.
Neurophysiological techniques can directly assess the functional state of specific cerebral regions and are indirect measures of local ischemia, which can be continuously monitored during endovascular aneurysm treatment. Several studies have documented the correlation between the NPM changes and cerebral ischemia. In patients undergoing carotid endarterectomy, Sharbrough et al (6) and Sundt et al (7, 8) found that major EEG changes occurred with rCBF <10 mL/100 g/min, with the critical level defined as 15 mL/100 g/min. Primate models show that SSEPs are maintained at levels of rCBF >16 mL/100 g/min but are absent at levels of rCBF <12 mL/100 g/min, with sharp reductions in the cortical SSEP amplitude (50% of baseline level) at rCBF levels between 14 and 16 mL/100 g/min (3, 5, 19, 20). Central conduction time is also prolonged with cerebral ischemia at an rCBF threshold of approximately 15 mL/100 g/min (21, 22). Still other primate studies have shown that cerebral infarction occurred at a rCBF threshold of 12 mL/100 g/min maintained for >2 hr (23, 24). These findings suggest that a 50% reduction in amplitude of the SSEP or a prolonged central conduction time >10 ms corresponds to an rCBF of 14 to 16 mL/100 g/min and is indicative of ischemia and possible progression to infarction. The usefulness of NPM has also been shown during surgical treatment of cerebral aneurysms (25).
This study evaluates the use of NPM to detect ischemia and alter management decisions during endovascular treatment of cerebral aneurysms; as such, it does not focus on clinical outcomes. Most of the patients in this study were deemed poor surgical candidates. Clinical outcomes were primarily influenced by the patient’s baseline state, including preexisting medical condition, by atherosclerotic disease in the cerebrovascular circulation, and by complication of the primary disease, such as severe subarachnoid hemorrhage and/or vasospasm. NPM changes altered treatment decisions for 14% of our patients (five of 35 patients). None of these five patients developed postprocedural neurologic deficits due to ischemia. In another 11% of the patients (four of 35 patients), treatment decisions were not altered despite NPM changes because an aneurysm rupture had occurred (three cases), requiring further GDC embolization of the aneurysm, or because diffuse NPM changes due to severe cerebral vasospasm were present.
The six ischemic events that resulted in permanent mild or moderate neurologic deficits in the study population were delayed complications occurring 15 hr to 5 days after therapy. Pelz et al (26) reported a 28% incidence of thromboembolic complications after GDC embolization, with a 5% incidence of permanent neurologic deficits. These are recognized complications for both GDC embolization and parent artery occlusion occurring after the procedure and do not diminish the potential value of NPM, which detects ischemic changes during the procedure.
We advocate a multimodality approach to NPM. The type of NPM is determined by the location of the aneurysm and its associated vascular territory, and the setup is critical. A complete description of the neurophysiological monitoring techniques is beyond the scope of this article but is described in a review article by Lopez (27). We use a combination of EEG, SSEPs, and BAEPs in the neuroangiography suite. The combination used is dependent on the vascular territory at risk and the patient’s baseline neurologic examination. Each technique has advantages and disadvantages, and it is best to use complementary modalities in combination.
The two cases in our study in which neurologic deficits were identified based on physical examination without corresponding NPM emphasize that NPM should not serve as a substitute but rather a complement to concurrent clinical neurologic testing whenever possible. We speculate, however, that NPM changes lagged behind the physical examination findings and would have subsequently become evident had our management not been altered. Unfortunately, the NPM literature does not yet address the issue of temporal correlation between onset of NPM changes and onset of neurologic deficits. In our study, several cases showed neurophysiological changes immediately after balloon inflation or attempted coil deployment. The literature also does not provide a definitive answer regarding how long NPM changes can persist before ischemia progresses to infarction. One study evaluated temporary occlusion in aneurysm surgery (28). In this study, the mean occlusion time was 20.3 min for the patients with middle cerebral artery aneurysms and 15.8 min for the patients with internal carotid artery aneurysms. The SSEP disappeared during occlusion in 42 of 97 patients (30 middle cerebral and 12 internal carotid arteries). All except three eventually returned to baseline after recirculation, and none of the 39 patients experienced postoperative sequelae. The time period from the start of occlusion until the complete loss of the SSEP averaged 8.6 min among these 39 patients, and the occlusion time from total SSEP loss until recirculation averaged 12 min. For the remaining three of the 42 patients (all three of whom had internal carotid artery aneurysms), however, the SSEP did not recover after recirculation, and all three patients experienced postoperative sequelae.
One limitation of NPM is that it may be relatively insensitive in detecting ischemic changes in some vascular territories, such as in the cerebellum or posterior cerebral artery territories, because NPM monitors the integrity of only specific sensory pathways and is dependent on somatosensory cortex and afferent pathways to generate the evoked response. SSEPs that use median and posterior tibial nerve stimulation are useful in assessing the functional state of the middle and anterior cerebral artery territories (by detecting impairment along the respective somatosensory pathways). BAEP monitoring detects functional changes along the auditory brain stem pathways. This is most often caused by a brain stem insult, which could result from vertebrobasilar ischemia. However, ischemia in the cerebellum or posterior cerebral artery territories could still be missed. EEG provides a more global assessment of cerebral ischemia but cannot be used to monitor the posterior fossa. It may be used to detect ischemia in the posterior cerebral artery territory, but its sensitivity is limited. Although the sensitivity of EEG could potentially be improved by increasing the number of electrodes used for monitoring, only a limited number of electrodes can be placed on the patient’s head (usually six) because the procedure is performed under fluoroscopy and more electrodes would obscure optimal working projections for endovascular treatment). In addition, the most commonly used monitoring equipment accepts only a limited number of electrode inputs.
Other technical limitations include confounding anesthesia-related effects, which may mimic cerebral ischemia. The effects of specific anesthetic agents are discussed in detail in the review article by Lopez (27). These effects can generally be distinguished from true ischemia by recognizing typical waveform characteristics (eg, bilateral rather than unilateral waveform changes) and by recognizing changes either in the type or dose of anesthetic agent used. Finally, evoked potential changes cannot be monitored at the time of coil release because of the nature of the electrolytic coil detachment, which interferes with the acquisition of the evoked potential. However, evoked potentials can be measured when the decision to detach the coil is made and can be monitored afterward. Also, with the advent of more rapidly detaching electrolytic coils, this drawback has been further diminished. Despite these limitations, we think that our experience shows the value of routine NPM during endovascular treatment of cerebral aneurysms, with NPM changes detected for 26% of patients treated and with NPM changes altering treatment decisions for 14%.
NPM is performed under the direction of one of the neurologists in the Department of Neurology and Neurologic Sciences. The neurologist supervising the NPM will typically examine the patient preoperatively and also perform a physical examination. The NPM team is composed of one neurodiagnostic technologist, who was originally trained as an EEG and evoked potential technologist and later received specialized training in intraoperative monitoring, and a neurologist trained in clinical neurophysiology and preferably intraoperative monitoring. An EEG/evoked potential machine is used to record the electrophysiological signals during the case. It is usual for the technologist to remain in the room for the duration of the case and for the neurologist to be present at the beginning of the procedure, during the time of balloon occlusion, and at the end of the case. The cost incurred for NPM includes an hourly hospital technical and physician professional fee. At our institution, this has been estimated to be approximately 10% of the cost of the neurointerventional procedure. NPM does not significantly prolong the interventional procedure because most of the patient electrode setup and the preoperative neurologic examination have been performed before the patient arrives in the radiologic suite. In addition, the time required to obtain baseline evoked potentials and EEG is approximately 5 min. NPM performed during the endovascular procedure usually does not require that the embolization procedure be interrupted.
In conclusion, NPM is a valuable adjunct to endovascular treatment of cerebral aneurysms. NPM may be particularly useful in situations in which neurologic examination is not possible (such as when the patient is under general anesthesia) or when a patient’s condition (such as obtunded subarachnoid hemorrhage) precludes neurologic testing. Our study suggests that these monitoring techniques may reduce ischemic complications and can be used to help guide therapeutic decisions.
References
- 1.Mathis JM, Barr JD, Jungreis CA, et al. Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am J Neuroradiol 1995;16:749–754 [PMC free article] [PubMed] [Google Scholar]
- 2.Sundt TM Jr, Michenfelder JD. Focal transient cerebral ischemia in the squirrel monkey: effect on brain adenosine triphosphate and lactate levels with electrocorticographic and pathologic correlation. Circ Res 1972;30:703–712 [DOI] [PubMed] [Google Scholar]
- 3.Branston NM, Symon L, Crockard HA, Pasztor E. Relationship between the cortical evoked potential and local cortical blood flow following acute middle cerebral artery occlusion in the baboon. Exp Neurol 1974;45:195–208 [DOI] [PubMed] [Google Scholar]
- 4.Branston NM, Strong AJ, Symon L. Extracellular potassium activity, evoked potential and tissue blood flow: relationships during progressive ischaemia in baboon cerebral cortex. J Neurol Sci 1977;32:305–321 [DOI] [PubMed] [Google Scholar]
- 5.Branston NM, Ladds A, Symon L, Wang AD. Comparison of the effects of ischaemia on early components of the somatosensory evoked potential in brainstem, thalamus, and cerebral cortex. J Cereb Blood Flow Metab 1984;4:68–81 [DOI] [PubMed] [Google Scholar]
- 6.Sharbrough FW, Messick JM Jr, Sundt TM Jr. Correlation of continuous electroencephalograms with cerebral blood flow measurements during carotid endarterectomy. Stroke 1973;4:674–683 [DOI] [PubMed] [Google Scholar]
- 7.Sundt TM Jr, Sharbrough FW, Anderson RE, Michenfelder JD. Cerebral blood flow measurements and electroencephalograms during carotid endarterectomy. J Neurosurg 1974;41:310–320 [DOI] [PubMed] [Google Scholar]
- 8.Sundt TM Jr, Sharbrough FW, Piepgras DG, Kearns TP, Messick JM Jr, O’Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc 1981;56:533–543 [PubMed] [Google Scholar]
- 9.Hacke W, Zeumer H, Ringelstein EB. EEG controlled occlusion of the internal carotid artery during angiography. Neuroradiology 1981;22:19–22 [DOI] [PubMed] [Google Scholar]
- 10.Hacke W, Zeumer H, Berg-Dammer E. Monitoring of hemispheric or brainstem functions with neurophysiologic methods during interventional neuroradiology. AJNR Am J Neuroradiol 1983;4:382–384 [PMC free article] [PubMed] [Google Scholar]
- 11.Hacke W, Berg-Dammer E, Zeumer H. Evoked potential monitoring during acute occlusion of the basilar artery and selective local thrombolytic therapy. Arch Psychiatr Nervenkr 1983;232:541–548 [DOI] [PubMed] [Google Scholar]
- 12.Ferbert A, Buchner H, Bruckmann H, Zeumer H, Hacke W. Evoked potentials in basilar artery thrombosis: correlation with clinical and angiographic findings. Electroencephalogr Clin Neurophysiol 1988;69:136–147 [DOI] [PubMed] [Google Scholar]
- 13.Zentner J, Schumacher M, Bien S. Motor evoked potentials during interventional neuroradiology. Neuroradiology 1988;30:252–255 [DOI] [PubMed] [Google Scholar]
- 14.Cloughesy TF, Nuwer MR, Hoch D, Viñuela F, Duckwiler G, Martin N. Monitoring carotid test occlusions with continuous EEG and clinical examination. J Clin Neurophysiol 1993;10:363–369 [DOI] [PubMed] [Google Scholar]
- 15.Anderson LC, Hemler DE, Luethke JM, Latchaw RE. Transcranial magnetic evoked potentials used to monitor the spinal cord during neuroradiologic angiography of the spine. Spine 1994;19:613–616 [DOI] [PubMed] [Google Scholar]
- 16.Paiva T, Campos J, Baeta E, Gomes LB, Martins IP, Parreira E. EEG monitoring during endovascular embolization of cerebral arteriovenous malformations. Electroencephalogr Clin Neurophysiol 1995;95:3–13 [DOI] [PubMed] [Google Scholar]
- 17.Paulsen RD, Steinberg GK, Norbash AM, Marcellus ML, Lopez JR, Marks MP. Embolization of rolandic cortex arteriovenous malformations. Neurosurgery 1999;44:479–486 [DOI] [PubMed] [Google Scholar]
- 18.Paulsen RD, Steinberg GK, Norbash AM, Marcellus ML, Marks MP. Embolization of basal ganglia and thalamic arteriovenous malformations. Neurosurgery 1999;44:991–997 [DOI] [PubMed] [Google Scholar]
- 19.Lesnick JE, Michele JJ, Simeone FA, DeFeo S, Welsh FA. Alteration of somatosensory evoked potentials in response to global ischemia. J Neurosurg 1984;60:490–494 [DOI] [PubMed] [Google Scholar]
- 20.Symon L. The relationship between CBF, evoked potentials and the clinical features in cerebral ischaemia. Acta Neurol Scand Suppl 1980;78:175–190 [PubMed] [Google Scholar]
- 21.Symon L, Crockard HA, Dorsch NW, Branston NM, Juhasz J. Local cerebral blood flow and vascular reactivity in a chronic stable stroke in baboons. Stroke 1975;6:482–492 [DOI] [PubMed] [Google Scholar]
- 22.Hargadine JR, Branston NM, Symon L. Central conduction time in primate brain ischemia: a study in baboons. Stroke 1980;11:637–642 [DOI] [PubMed] [Google Scholar]
- 23.Morawetz RB, DeGirolami U, Ojemann RG, Marcoux FW, Crowell RM. Cerebral blood flow determined by hydrogen clearance during middle cerebral artery occlusion in unanesthetized monkeys. Stroke 1978;9:143–149 [DOI] [PubMed] [Google Scholar]
- 24.Jones TH, Morawetz RB, Crowell RM, et al. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg 1981;54:773–782 [DOI] [PubMed] [Google Scholar]
- 25.Lopez JR, Chang SD, Steinberg GK. The use of electrophysiological monitoring in the intraoperative management of intracranial aneurysms. J Neurol Neurosurg Psychiatry 1999;66:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelz DM, Lownie SP, Fox AJ. Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol 1998;19:1541–1547 [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez R. Intraoperative neurophysiological monitoring. Int Anesthesiol Clin 1996;34:33–54 [DOI] [PubMed] [Google Scholar]
- 28.Mizoi K, Yoshimoto T. Permissible temporary occlusion time in aneurysm surgery as evaluated by evoked potential monitoring. Neurosurgery 1993;33:434–440 [DOI] [PubMed] [Google Scholar]