Abstract
BACKGROUND AND PURPOSE: Recent interest has emerged in the use of pharmacologic methods to maximize blood oxygenation level-dependent (BOLD) signal intensity changes in functional MR imaging (fMRI). Adenosine antagonists, such as caffeine and theophylline, have been identified as potential agents for this purpose. The present study was designed to determine whether caffeine-induced decreases in cerebral perfusion result in enhanced BOLD responses to visual and auditory stimuli.
METHODS: MR imaging was used to measure resting cerebral perfusion and stimulus-induced BOLD signal intensity changes in 19 patients. We evaluated the relationship between resting cerebral perfusion and the magnitude of BOLD signal intensity induced by visual and auditory stimulation under caffeine and placebo conditions.
RESULTS: The data showed that changes in resting cerebral perfusion produced by caffeine are not a consistent predictor of BOLD signal intensity magnitude. Although all cerebral perfusion was reduced in all study participants in response to caffeine, only 47% of the participants experienced BOLD signal intensity increase. This finding was independent of the participants’ usual caffeine consumption.
CONCLUSION: The data presented herein show that the relationship between resting cerebral perfusion and the magnitude of BOLD signal intensity is complex. It is not possible to consistently enhance BOLD signal intensity magnitude by decreasing resting perfusion with caffeine. Future studies aimed at evaluating the relationship between perfusion and BOLD signal intensity changes should seek a means to selectively modulate known components of the neural and vascular responses independently.
Functional MR imaging (fMRI) is a technique with which to measure neural activity based on changes in blood oxygenation (1, 2). In an area of brain activity, vasoactive substances are released and produce an increase in regional blood flow. However, functional MR imaging is not directly sensitive to this change in blood flow but rather to changes in the level of oxygenation that accompany the increased blood flow. The exact mechanisms involved in the coupling of neural activity to regional changes in blood flow and the subsequent change in the blood oxygenation level-dependent (BOLD) signal intensity are just beginning to be elucidated (3, 4). However, strong evidence exists to suggest that at least adenosine and nitric oxide are major contributors to the regional vasodilation responsible for this phenomenon (5, 6). Several studies have clearly shown that agonists and antagonists of the adenosine and nitric oxide receptor systems can modulate resting perfusion as well as functional MR imaging and positron emission tomography can measure activity-induced changes in blood flow (7–10).
Historically, the signal intensity change in a typical fMRI study is approximately 1% to 5%, but recent studies of complex cognitive processes have observed more subtle BOLD signal intensity changes, often <1%. Attempts have been made to find tools that can be used to enhance BOLD signal intensity (11). Many investigators have studied the effects of methylxanthines, adenosine receptor antagonists, because these drugs are readily available, are safe, and have been studied extensively (7–10, 12–15). However, the methylxanthines, such as caffeine, are nonselective adenosine receptor antagonists and block neurovascular receptors (predominantly A2) with a slightly higher affinity than that of neural receptors (predominantly A1) (13, 16). Blockade of vascular adenosine receptors produces vasoconstriction and decreases resting cerebral perfusion (13, 17–20). It has been suggested that this decrease in baseline cerebral perfusion can produce an enhanced BOLD signal intensity (9, 10, 21, 22).
As previously noted, the CNS effects of the methylxanthines are not limited to changes in the neurovasculature. Adenosine receptors are also located on neurons and are inhibitory in nature. Thus, an antagonist of these receptors has stimulant properties through disinhibitory mechanisms (13, 23, 24). One can then see the complicated nature of the system and the problems that may occur when trying to use adenosine antagonists to boost BOLD signal intensity. A nonselective adenosine antagonist can have both neural and vascular effects depending on the ratio of A1 to A2 receptors in any given brain region, study population, or individual person (5). The role of adenosine receptors in BOLD signal intensity change remains unresolved, as does modulation of the resting perfusion to influence the magnitude of BOLD signal intensity (5, 7, 9, 11, 21, 22, 25–27). The present study was designed to evaluate the correlation between resting cerebral perfusion and the magnitude of visual and auditory induced BOLD signal intensity changes associated with caffeine and placebo administration.
Methods
The methods used for the collection and analysis of data for the individual perfusion and fMRI studies have been reported previously (8, 12). The data presented herein represent the results of further analysis of the data from those two studies. Pertinent methods are reviewed below.
Study Participants and Study Design
Twenty healthy adult volunteers (16 men, four women; age range, 24–64 years) participated in the study after meeting the following inclusion criteria: no history of migraine, stroke, hypertension, diabetes, or any neurologic or vascular disease; no history of alcohol or drug abuse; and no use of tobacco products or oral contraceptives. Study participants were categorized as low (<120 mg/day, 10 participants) or moderate to high (>300 mg/day, 10 participants) caffeine users based on their responses to a dietary questionnaire and published data on the caffeine content of common beverages (28). After receiving an explanation of the study procedure, participants provided written informed consent approved by the Institutional Review Board for Human Subjects at our institution.
A perfusion imaging sequence (8) and two fMRI scans (12) were obtained for each participant; repeat images were obtained on two different days. Participants were randomized to receive caffeine (250 mg orally, equivalent to approximately two cups of coffee) or placebo (250 mg lactose capsule) on each of the 2 days in a single blind, counterbalanced design. For the fMRI experiments, participants were presented with a passive visual (flashing checkerboard) and a passive auditory (bursts of white noise) epoch-based paradigm.
Image Acquisition
All experiments were conducted on a 1.5-T GE Echo-speed Horizon LX imaging unit with a birdcage head coil (GE Medical Systems, Milwaukee, WI). A relative cerebral blow flow image and a T1 map were obtained by using a flow-sensitive alternating inversion recovery sequence on a single axial section parallel and 10 mm cephalad to the anteroposterior commissural line. The sequence consisted of alternating section-selective and nonselective RF inversion pulses, a diffusion gradient (equivalent b value, 5.25 mm2/s) for suppression of intra-arterial spins, and a single shot spiral readout gradient. A hyperbolic secant pulse was used for the RF inversion. The width of the section-selective inversion pulse was 20 mm wider than the imaging section (10 mm on either side). A quantitative cerebral blow flow image was then calculated from the relative cerebral blow flow and T1 images by using a published perfusion model (29).
Whole brain activation was assessed by examining BOLD changes in T2* relaxation rate that accompanied cortical activation (2, 30). Functional imaging was performed in the axial plane by using multisection gradient-echo echo-planar imaging with a field of view of 24 cm (frequency) × 15 cm (phase) and an acquisition matrix of 64 × 40 (28 sections, 5-mm thickness, no skip, 2500/40 [TR/TE]). High resolution structural images were obtained by using a 3D spoiled gradient-echo sequence with the following parameters: matrix, 256 × 256; field of view, 24 cm; section thickness, 3 mm with no gap between sections; number of sections, 60; in-plane resolution, 0.94 mm.
Image Processing
Cerebral blow flow data were first segmented into gray and white matter regions by using SPM99. Mean cerebral blow flow values were then calculated separately for the white and gray matter regions. Whole brain gray matter perfusion values are presented herein.
The fMRI data were processed by using SPM99 (31, 32) from the Wellcome Department of Cognitive Neurology, London, England, implemented in Matlab (The Mathworks Inc., Sherborn, MA) with an IDL (Research Systems Inc., Boulder, CO) interface. Before generating statistical parametric maps, data were motion corrected within SPM99 (33), normalized to Montreal Neurologic Institute space by using image header information (34) in combination with the SPM99 normalization (33), and resampled to 4 × 4 × 5 mm by using sinc interpolation. The data sets were smoothed by using an 8 × 8 × 10 mm full-width-half-maximum gaussian kernel. The data were modeled with a boxcar design convolved with a standardized hemodynamic response function. Global normalization, temporal smoothing, detrending, and high pass filtering were performed as part of the statistical parametric map analysis. One low user was excluded from the final analyses because of excessive head motion that was correlated with the stimulus paradigm (35).
Perfusion/fMRI Combined Analysis
Total fMRI response magnitude was calculated for each participant for each condition by multiplying the mean percent signal intensity change in each significantly activated cluster (P ≤ .05 extent corrected) by the cluster volume. In the event that no clusters survived correction for multiple comparisons, the total fMRI response of zero was used. This situation was encountered for only four of the total 76 runs. The difference values for the fMRI and perfusion measures were calculated by subtracting the placebo condition from the caffeine condition. The difference measures for response magnitude and resting perfusion were analyzed by using linear regression.
Results
It has been proposed that decreasing resting cerebral perfusion could result in increased BOLD signal intensity. To evaluate this possibility, we compared the perfusion difference between the caffeine and placebo conditions to the visual and auditory BOLD signal intensity changes under the same drug conditions. Because all participants experienced a decrease in cerebral perfusion in response to caffeine (Table 1) (8), we could determine whether the caffeine-induced decrease in resting perfusion was correlated with an increase in BOLD signal intensity. No significant correlation was found between the magnitude of the caffeine-induced decrease in cerebral perfusion and the BOLD signal intensity difference between the caffeine and placebo conditions in either visual or auditory cortex (Fig 1). Only 47% of the participants experienced an increase in BOLD signal intensity in the lower resting perfusion state. The other 53% experienced a decrease in the magnitude of BOLD signal intensity with lower resting perfusion rates. Linear regression revealed no significant correlation between changes in cerebral perfusion and BOLD signal intensity. In addition, when evaluated independently, neither high nor low caffeine users exhibited significant correlation between change in perfusion and BOLD signal intensity change. These observations were consistent for responses to both auditory and visual stimulation.
Total blood oxygen level-dependent signal changes, cerebral perfusion changes, and difference values for placebo and caffeine conditions*
| Participant No. | Visual BOLD Change (mean % change × cluster volume) |
Auditory BOLD Change (mean % change × cluster volume) |
Gray Matter Perfusion (mL/100 g of tissue/min) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Caffeine | Placebo | Difference | Caffeine | Placebo | Difference | Caffeine | Placebo | Difference | |
| 1 | 460.48 | 473.76 | −13.28 | 398.8 | 492.19 | −93.39 | 45.6 | 49.0 | −3.4 |
| 2 | 445.67 | 465.18 | −19.51 | 213.13 | 250.47 | −37.34 | 45.0 | 57.2 | −12.3 |
| 3 | 572.42 | 632.36 | −59.94 | 321.08 | 216.46 | 104.62 | 48.8 | 56.9 | −8.1 |
| 4 | 308.05 | 33.6 | 274.45 | 185.04 | 90.13 | 94.91 | 42.3 | 61.4 | −19.1 |
| 5 | 380.64 | 574.3 | −193.66 | 0 | 323.81 | −323.81 | 55.3 | 70.5 | −15.2 |
| 6 | 347.7 | 243.24 | 104.46 | 264.02 | 396.66 | −132.64 | 36.7 | 40.1 | −3.4 |
| 7 | 433.04 | 655.2 | −222.16 | 26.64 | 89.86 | −63.22 | 61.5 | 68.5 | −7.0 |
| 8 | 304.92 | 785.24 | −480.32 | 561.84 | 255.04 | 306.8 | 38.5 | 51.5 | −13.1 |
| 9 | 272.4 | 357.7 | −85.3 | 38.48 | 278.88 | −240.4 | 43.5 | 70.6 | −27.1 |
| 10 | 697.74 | 583.27 | 114.47 | 313.02 | 115.78 | 197.24 | 60.5 | 80.7 | −20.2 |
| 11 | 815.28 | 446.76 | 368.52 | 91.54 | 84.24 | 7.3 | 48.6 | 66.3 | −17.7 |
| 12 | 621.16 | 680.56 | −59.4 | 311.75 | 416.22 | −104.47 | 53.1 | 64.6 | −11.5 |
| 13 | 566.62 | 936.39 | −369.77 | 158.44 | 0 | 158.44 | 54.5 | 72.6 | −18.1 |
| 14 | 803.08 | 521.64 | 281.44 | 452.15 | 100.62 | 351.53 | 53.9 | 64.6 | −10.7 |
| 15 | 242.08 | 519.68 | −277.6 | 110.55 | 374.44 | −263.89 | 40.1 | 63.0 | −22.9 |
| 16 | 813.77 | 391.14 | 422.63 | 280.28 | 247.24 | 33.04 | 56.1 | 68.6 | −12.5 |
| 17 | 792.57 | 590.72 | 201.85 | 127.38 | 156.22 | −28.84 | 68.5 | 92.4 | −23.9 |
| 18 | 666.3 | 624.26 | 42.04 | 132.48 | 84.76 | 47.72 | 56.9 | 82.5 | −25.6 |
| 19 | 373.23 | 0 | 373.23 | 258.78 | 0 | 258.78 | 60.0 | 88.3 | −28.3 |
Note.—BOLD indicates blood oxygen level-dependent.
The blood oxygen level-dependent change was calculated by multiplying the mean percent change by the number of voxels in the cluster. This calculation was used to account for signal change magnitude and activation volume. The zeros in the blood oxygen level-dependent columns represent cases in which, although activation was present, the activation clusters did not survive correction for multiple comparisons.
Fig 1.
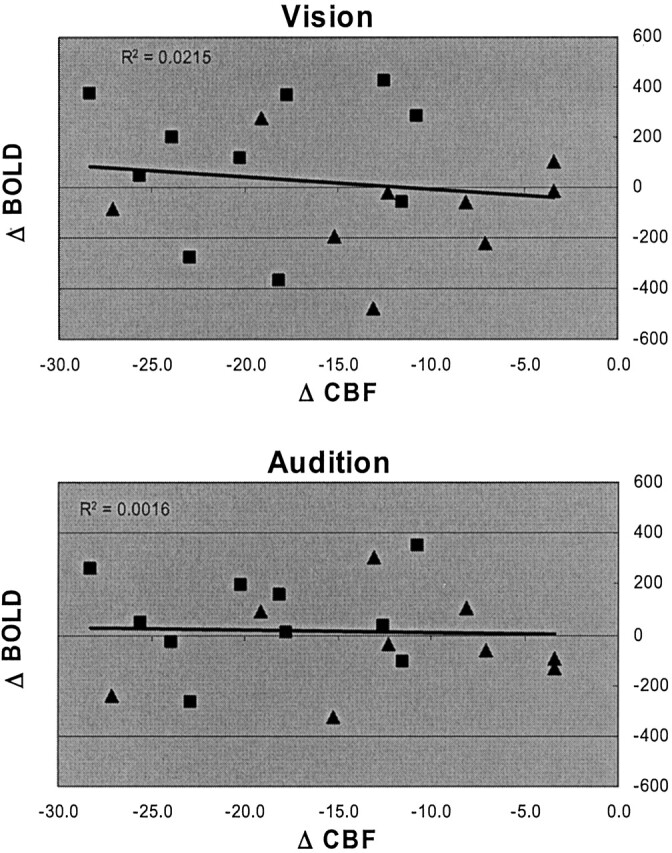
Difference correlation plots for visual and auditory conditions. These graphs were generated by subtracting CBF and BOLD measurements made in the placebo condition from those made in the caffeine condition. All values on the perfusion difference axes (Δ CBF) are negative because resting cerebral perfusion decreased for all study participants when the caffeine condition was compared with the placebo condition. The values on the BOLD signal difference axes (Δ BOLD) are positive if BOLD signal increased when perfusion decreased and are negative if BOLD signal decreased when perfusion decreased. It is evident that half of the population experienced an increase and the other half a decrease in BOLD signal when resting perfusion decreased. Data are shown separately for the high (squares) and low (triangles) caffeine users. The regression lines were calculated for the population as a whole and show the poor correlation between the magnitude of the perfusion decrease and the BOLD signal change in visual and auditory cortex. The units are mL/100 g of brain tissue/min for Δ CBF and total percent change (mean percent change × cluster volume) for Δ BOLD.
Discussion
Much debate has recently been presented regarding the effects of resting cerebral perfusion on the magnitude of BOLD signal intensity (7, 9, 11, 21, 26, 36). Studies of both humans and animal models have suggested that decreases in resting cerebral perfusion, typically achieved by using adenosine antagonists, can produce increases in the magnitude of evoked BOLD signal intensity changes. It has been proposed that the increased BOLD signal intensity is due to an increase in the difference between the resting and the active cerebral perfusion (9, 11). With the present study, we evaluated the relationship between resting cerebral perfusion and BOLD signal intensity change under caffeine and placebo conditions.
The results presented herein show that caffeine-induced decreases in resting cerebral perfusion in each participant resulted in no predictable changes in BOLD signal intensity magnitude, with approximately half of our study population showing decreases and half showing increases. These findings show that the relationship between resting cerebral perfusion and BOLD signal intensity changes is complex. Because BOLD signal intensity is dependent on both neural and vascular responses, any physiological condition or drug that influences both of these components is destined to produce complex changes in the neurovascular coupling underlying BOLD signal intensity. For example, any drug that increases stimulus-induced neural activity independent of the baseline neural activity has the potential to increase BOLD signal intensity. Similarly, any drug that reduces resting neuronal activity but has no effect on stimulus-induced neuronal firing will increase the BOLD signal intensity difference between baseline and active conditions. However, a drug that increases baseline neural activity or decreases stimulus-induced activity would be expected to decrease the BOLD signal intensity difference between resting and active states.
Similar complexities arise when considering compounds with vascular reactivity. A drug that decreases resting perfusion could increase BOLD signal intensity if the compound does not also block the receptors that are responsible for the vascular component of BOLD signal intensity. It has been reported that indomethacin reduces resting perfusion and increases stimulus-induced BOLD signal intensity changes (21). If the drug of interest reduces resting perfusion through a blockade of receptors important in generating BOLD signal intensity (ie, caffeine blockade of adenosine receptors), these receptors will not be available to produce vasodilation during regional neural activity and BOLD signal intensity will be attenuated. Interestingly, it is also likely that drugs that increase resting cerebral perfusion will decrease BOLD signal intensity. By producing vasodilation, the net vascular reserve available for regional increases in cerebral perfusion diminishes, producing a decrease in the maximal BOLD signal intensity attainable. Thus, drugs such as acetazolamide increase resting cerebral perfusion and decrease the BOLD response (36).
It should be noted that for the current study, we used single slice perfusion measures because of technical limitations. We were, therefore, unable to evaluate the relationship between resting perfusion and BOLD signal intensity in the exact same voxels. To compensate for this, we compared global changes in cerebral perfusion to regional BOLD changes in two separate cortical areas. The results in visual and auditory cortices were nearly identical. Future studies using whole brain perfusion techniques may be able to further elucidate the relationship between cerebral blow flow and BOLD signal intensity by comparing regional resting perfusion to regional BOLD signal intensity changes.
Conclusion
The data presented herein show that the relationship between resting cerebral perfusion and the magnitude of BOLD signal intensity is complex. It is not possible to consistently enhance BOLD signal intensity magnitude by simply decreasing resting perfusion. We propose that any drug or condition that has both vascular and neural effects will result in a complex relationship between resting cerebral perfusion and BOLD signal intensity changes. For example, the vascular effects of the methylxanthines result in a decrease in resting cerebral perfusion and likely attenuate BOLD signal intensity. However, the neural effects of these compounds may result in an increase in BOLD signal intensity through their neurostimulant effects. Thus, the net effects of the methylxanthines are due to a combination of the neural and vascular responses, which are dependent on many factors, including receptor number and affinity. Future studies aimed at evaluating the relationship between perfusion and BOLD signal intensity changes should seek a means to selectively modulate known components of the neural and vascular responses independently.
References
- 1.Ogawa S, Tank DW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A 1992;89:5951–5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990;87:9868–9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001;412:150–157 [DOI] [PubMed] [Google Scholar]
- 4.Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal? Trends Neurosci 2002;25:27–31 [DOI] [PubMed] [Google Scholar]
- 5.Gotoh J, Kuang TY, Nakao Y, et al. Regional differences in mechanisms of cerebral circulatory response to neuronal activation. Am J Physiol Heart Circ Physiol 2001;280:H821–H829 [DOI] [PubMed] [Google Scholar]
- 6.Cholet N, Seylaz J, Lacombe P, Bonvento G. Local uncoupling of the cerebrovascular and metabolic responses to somatosensory stimulation after neuronal nitric oxide synthase inhibition. J Cereb Blood Flow Metab 1997;17:1191–1201 [DOI] [PubMed] [Google Scholar]
- 7.Morton DW, Maravilla KR, Meno JR, Winn HR. Systemic theophylline augments the blood oxygen level-dependent response to forepaw stimulation in rats. AJNR Am J Neuroradiol 2002;23:588–593 [PMC free article] [PubMed] [Google Scholar]
- 8.Field AS, Laurienti PJ, Yen YF, Burdette JH, Moody DM. Dietary caffeine consumption and withdrawal: confounding variables in quantitative cerebral perfusion studies? Radiology 2003;227:129–135 [DOI] [PubMed] [Google Scholar]
- 9.Mulderink TA, Gitelman DR, Mesulam MM, Parrish TB. On the use of caffeine as a contrast booster for BOLD fMRI studies. Neuroimage 2002;15:37–44 [DOI] [PubMed] [Google Scholar]
- 10.Dager SR, Friedman SD. Brain imaging and the effects of caffeine and nicotine. Ann Med 2000;32:592–599 [DOI] [PubMed] [Google Scholar]
- 11.Li TQ, Mathews VP. How can we make BOLD contrast bolder? AJNR Am J Neuroradiology 2002;23:507–508 [PMC free article] [PubMed] [Google Scholar]
- 12.Laurienti PJ, Field AS, Burdette JH, Maldjian JA, Yen YF, Moody DM. Dietary caffeine consumption modulates fMRI measures. Neuroimage 2002;17:751–757 [PubMed] [Google Scholar]
- 13.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 2001;24:31–55 [DOI] [PubMed] [Google Scholar]
- 14.Johansson B, Ahlberg S, van der Ploeg I, et al. Effect of long term caffeine treatment on A1 and A2 adenosine receptor binding and on mRNA levels in rat brain. Naunyn Schmiedebergs Arch Pharmacol 1993;347:407–414 [DOI] [PubMed] [Google Scholar]
- 15.Shi D, Daly JW. Chronic effects of xanthines on levels of central receptors in mice. Cell Mol Neurobiol 1999;19:719–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varani K, Portaluppi F, Gessi S, et al. Dose and time effects of caffeine intake on human platelet adenosine A(2A) receptors: functional and biochemical aspects. Circulation 2000;102:285–289 [DOI] [PubMed] [Google Scholar]
- 17.Moreau JL, Huber G. Central adenosine A(2A) receptors: an overview. Brain Res Brain Res Rev 1999;31:65–82 [DOI] [PubMed] [Google Scholar]
- 18.Shin HK, Park SN, Hong KW. Implication of adenosine A2A receptors in hypotension-induced vasodilation and cerebral blood flow autoregulation in rat pial arteries. Life Sci 2000;67:1435–1445 [DOI] [PubMed] [Google Scholar]
- 19.Ngai AC, Coyne EF, Meno JR, et al. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am J Physiol Heart Circ Physiol 2001;280:H2329–H2335 [DOI] [PubMed] [Google Scholar]
- 20.Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev 1997;49:381–402 [PubMed] [Google Scholar]
- 21.Bruhn H, Fransson P, Frahm J. Modulation of cerebral blood oxygenation by indomethacin: MRI at rest and functional brain activation. J Magn Reson Imaging 2001;13:325–334 [DOI] [PubMed] [Google Scholar]
- 22.Parrish T, Mulderink T, Gitelman D, Mesulam M. Caffeine as a bold contrast booster. Neuroimage 2001;13: Part 2 Suppl1001 [DOI] [PubMed] [Google Scholar]
- 23.Haas HL, Selbach O. Functions of neuronal adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol 2000;362:375–381 [DOI] [PubMed] [Google Scholar]
- 24.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 1998;50:413–492 [PubMed] [Google Scholar]
- 25.Kanno I, Shimosegawa E, Fujita H, Hatazawa J. Uncoupling of absolute CBF to neural activity. Adv Exp Med Biol 1997;413:209–214 [DOI] [PubMed] [Google Scholar]
- 26.Matsuura T, Fujita H, Kashikura K, Kanno I. Evoked local cerebral blood flow induced by somatosensory stimulation is proportional to the baseline flow. Neurosci Res 2000;38:341–348 [DOI] [PubMed] [Google Scholar]
- 27.Li TQ, Kastrup A, Moseley ME, Glover GH. Changes in baseline cerebral blood flow in humans do not influence regional cerebral blood flow response to photic stimulation. J Magn Reson Imaging 2000;12:757–762 [DOI] [PubMed] [Google Scholar]
- 28.Bunker ML, McWilliams M. Caffeine content of common beverages. J Am Diet Assoc 1979;74:28–32 [PubMed] [Google Scholar]
- 29.Yang Y, Frank JA, Hou L, Ye FQ, McLaughlin AC, Duyn JH. Multislice imaging of quantitative cerebral perfusion with pulsed arterial spin labeling. Magn Reson Med 1998;39:825–832 [DOI] [PubMed] [Google Scholar]
- 30.Ogawa S, Menon RS, Tank DW, et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging: a comparison of signal characteristics with a biophysical model. Biophys J 1993;64:803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage 1995;2:166–172 [DOI] [PubMed] [Google Scholar]
- 32.Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuroimage 1995;2:157–165 [DOI] [PubMed] [Google Scholar]
- 33.Friston KJ, Ashburner J, Poline J, et al. Spatial registration and normalization of images. Hum Brain Mapp 1995;2:165–189 [Google Scholar]
- 34.Maldjian JA, Schulder M, Liu WC, et al. Intraoperative functional MRI using a real-time neurosurgical navigation system. J Comput Assist Tomogr 1997;21:910–912 [DOI] [PubMed] [Google Scholar]
- 35.Field AS, Yen YF, Burdette JH, Elster AD. False cerebral activation on BOLD functional MR images: study of low-amplitude motion weakly correlated to stimulus. AJNR Am J Neuroradiol 2000;21:1388–1396 [PMC free article] [PubMed] [Google Scholar]
- 36.Bruhn H, Kleinschmidt A, Boecker H, Merboldt KD, Hanicke W, Frahm J. The effect of acetazolamide on regional cerebral blood oxygenation at rest and under stimulation as assessed by MRI. J Cereb Blood Flow Metab 1994;14:742–748 [DOI] [PubMed] [Google Scholar]


