Abstract
Background
Despite increasing neonatal antibiotic stewardship efforts, understanding of interhospital variation in neonatal antibiotic use is limited.
Methods
A retrospective cohort study was conducted among primarily academically affiliated hospitals participating in the Vizient Clinical Database/Resource Manager. Neonatal discharges were identified by admission age <1 month, excluding nonviable neonates and normal newborns. Hospitals with ≥100 neonatal discharges and complete data for January-December 2016 were included. Antibiotic use was measured in days of therapy per 1000 patient-days (DOT/1000 pd). A composite measure of neonatal care complexity (NCC; low, medium, high) was based on the volume of very low-birth-weight neonates and neonates undergoing surgical procedures, cardiac surgery, or extracorporeal membranous oxygenation.
Results
The 118 included hospitals represented 184 716 neonatal discharges; 22 hospitals with low NCC, 56 with medium NCC, and 40 with high NCC. Mean antibiotic DOT/1000 pd was 363 (standard deviation [SD], 94) in high NCC hospitals, 243 (SD, 88) in medium NCC hospitals, and 184 (SD, 122) in low NCC hospitals. Increasing NCC was associated with higher antibiotic use, with an incidence rate ratio (IRR) of 1.95 (95% confidence interval [CI], 1.55 to 2.47) for high vs low NCC and IRR 1.31 (95% CI, 1.05 to 1.64) for medium vs low NCC. Increasing case mix index was associated with higher antibiotic use (IRR 1.86 per unit increase; 95% CI, 1.50 to 2.31).
Conclusions
Aggregate antibiotic use among hospitalized neonates varies based on care complexity. Substantial variation despite stratification by complexity suggests incomplete risk adjustment and/or avoidable variation in care.
Keywords: antimicrobial stewardship, antibacterial agents, newborn infant, risk adjustment
Hospital-level neonatal antibiotic use in primarily academically affiliated hospitals is associated with complexity of care, as measured by the patient population characteristics and numerical case mix index. Unexplained variation suggests opportunity to strengthen antibiotic stewardship and risk adjustment methods.
Antibiotics are commonly used in hospitalized neonates [1, 2]. They are vital in treating infections; however, their use is also associated with adverse outcomes including multidrug-resistant infections, necrotizing enterocolitis, late-onset sepsis, invasive candidiasis, and mortality [3–6]. Substantial variation in neonatal antibiotic use has been described and is not fully explained by burden of proven infection [7–11]. These factors have led to antibiotic stewardship efforts to improve judicious use of antibiotics in neonates.
Interhospital comparison of risk-adjusted antibiotic use is recommended by antibiotic stewardship implementation guidelines and national policy measures [12, 13]. However, at present, there is no validated model for interhospital comparison of neonatal antibiotic use. Current models for expected antibiotic use in the Centers for Disease Control and Prevention National Healthcare Safety Network Antibiotic Use (NHSN AU) module do not account for well newborn nurseries or neonatal intensive care units (NICUs); however, neonatal models are in development [14, 15]. Furthermore, while days of therapy per 1000 patient-days (DOT/1000 pd) is recommended to measure the impact of antibiotic stewardship programs, few studies to date have reported neonatal antibiotic use in DOT/1000 pd, presenting difficulty to hospitals that seek to compare to an external standard [16].
To inform appropriate comparison among hospitals and to characterize variation in hospital-level antibiotic use, we evaluated antibiotic use among hospitalized neonates in a national database of primarily academically affiliated hospitals. We hypothesized that a portion of variation in antibiotic use could be explained by measures of neonatal care complexity (NCC) measurable at the level of the hospital or the aggregated clinical population.
METHODS
Data Source and Study Population
A multicenter retrospective cohort study was conducted using data from the Vizient (formerly University Health System Consortium) Clinical Database-Resource Manager (CDB/RM, Irving, TX) [17]. Vizient is a performance improvement organization with member hospitals including 50% of acute care health systems and 95% of academic medical centers in the United States [18]. Vizient member hospitals are located in all major US geographic regions. A subset of member hospitals contribute data to the CDB-RM, a database that includes charge-based information on medications, procedures, and diagnoses for all discharged patients regardless of payer status. Most of the centers that contribute neonatal and/or pediatric data to CDB-RM are large academic medical centers with pediatric and/or neonatal care provided on a common facility license, rather than independently licensed children’s hospitals. The database has previously been used to benchmark antimicrobial use in adult and pediatric patients [19–21].
All data were aggregated at the hospital level but with the clinical population restricted to neonatal discharges during January 2016–December 2016. Neonatal discharges were defined based on admission age <1 month and could be admitted to any hospital location or from any admission source (eg, inborn, transfer, home). “Normal newborns” (defined by Medicare Severity Diagnosis Related Group [MS-DRG] 795) and nonviable neonates (at delivery) were excluded from the clinical population based on predefined selection criteria in the database [22]. “Normal newborns” were excluded in order to avoid introduction of unwarranted variation in the denominator of patient-days, given that the newborn delivery volume may vary widely between hospitals and not necessarily be related to factors that drive antibiotic use. The hospital was considered the primary unit of analysis for the study. Hospitals with complete CDB-RM data and at least 100 neonatal discharges (admission age <1 month) during the study period were included. A threshold for the number of discharges was set in order to exclude hospitals that may care for a small number of neonates admitted to pediatric wards and intensive care units but not routinely provide neonatal care. The study was designated exempt as not human subjects research by the University of California San Francisco Institutional Review Board.
Variable Definitions
Except where noted, all variables represent aggregate measures (means, counts, rates) for the included neonatal cases (with each case representing a neonatal discharge, excluding nonviable neonates and normal newborn discharges as outlined above) in each hospital. The primary outcome was antibiotic use measured by DOT/1000 pd [20]. The term “antibiotic” herein refers to systemic antibacterial medications, excluding nonsystemic routes and antiviral and antifungal agents. Antibiotics administered enterally were included, which is consistent with the current approach to measurement of antibiotics in the NHSN AU module [15]. Each antibiotic given on a calendar day contributed 1 DOT; for example, ampicillin and gentamicin given on 2 calendar days is 4 DOT. The total DOT for the population of patients is normalized to the patient census in 1000 patient-days (the average length of stay [LOS] multiplied by the number of cases). Antibiotic use data in CDB-RM have been previously validated against member hospital medication administration data [19]. The DOT/1000 pd was also determined for each of the most frequently used individual antibiotics. Secondary outcomes were complementary antibiotic use measures including percentage of cases who received an antibiotic, mean days of treatment with any antibiotic (antibiotic days [AD]), and antibiotic use rate (AUR; AD as a percentage of patient-days, previously used in the neonatal literature to report aggregate antibiotic use) [9]. The antibiotic spectrum index (ASI) per AD was calculated to capture the spectrum of activity using previously published consensus scoring, with a higher ASI indicating broader-spectrum exposure [23, 24]. The ASI assigns numerical values for an antibiotic that has activity against 1 or more of 13 categories of pathogen and an additional point for activity against multidrug-resistant organisms. It is totaled and then normalized to the number of days on antibiotic therapy (AD).
In order to determine whether hospital-level characteristics and complexity measures were associated with aggregate antibiotic use in neonates and develop candidate models for the expected amount of antibiotic use based on those characteristics, we examined the association between aggregate antibiotic DOT and predictor variables including US census region, total neonatal case volume (excluding normal newborns and nonviable neonates as above), and case mix index (CMI) for the included neonatal cases in each hospital. CMI, a measure of anticipated healthcare resource utilization, is calculated by weighted averaging of MS-DRGs [25]. The number of low birth-weight (BW) neonates by BW category (<1000 g, 1000–1499 g, and 1500–2499 g), number who underwent major surgical procedures, number of neonates with BW >2499 g who underwent cardiac surgical procedures, and number who received extracorporeal membrane oxygenation (ECMO) were derived from neonatal discharge All Patients Refined Diagnosis Groups (APR-DRGs). Inborn case numbers were determined based on admission source coded as “born in this hospital.”
Each hospital’s self-reported NICU level of care was obtained from the Vizient Hospital Profile Report. Because categories were frequently not reported, we derived post hoc NCC categories based on each hospital’s neonatal population characteristics, using criteria that corresponded to the American Academy of Pediatrics (AAP) levels of neonatal care [26]. These groupings were intended to create a composite variable that mimics NICU level of care. Hospitals with <5 cases with BW <1500 g (very low birth weight; VLBW), <5 cases with major surgical procedure(s), <5 cardiac surgery cases, and no ECMO cases were designated low NCC hospitals. Hospitals with ≥5 VLBW cases or ≥5 cases with major surgical procedure(s) but <5 cardiac surgery cases and no ECMO cases were designated medium NCC hospitals. Hospitals with ≥5 VLBW cases and either any ECMO cases or ≥5 cardiac surgery cases were designated high NCC hospitals. The threshold of <5 VLBW, major procedure, and/or cardiac cases was used to account for hospitals that provide initial stabilization or convalescent care for neonates in these groups but do not routinely offer such care. Because the number of neonates receiving ECMO is much smaller, centers with any ECMO cases were designated high NCC [27, 28].
Selected infectious events were estimated based on International Statistical Classification of Diseases and Related Health Problems, 10th revision, diagnostic codes for bacterial sepsis (P36, P36.0–36.5, P36.10, P36.19, P36.8, P36.9) and necrotizing enterocolitis (K5530–5533, P77, P771–773, P779) but were not evaluated as predictors of antibiotic use due to the inability to differentiate the temporal relationship between these events and antibiotic use from the available data. Similarly, average LOS and mortality were collected but not evaluated as predictors.
Statistical Analyses
Antibiotic use measures were compared across NCC categories using analysis of variance, followed by Tukey pairwise comparisons. Negative binomial regression models were developed incorporating DOT as a count outcome and patient-days as the exposure term, starting with single candidate predictors. Predictors with a statistically significant Wald test in the initial single predictor model were further evaluated in combination with one another. The modeling strategy was developed a priori based on hypothesized relationships between variables. Because we evaluated predictors that measure similar aspects of care complexity (eg, the number of VLBW cases is a component of NCC), we started by comparing 3 types of complexity measures for the initial candidate model and then tested whether additional predictors improved performance of the initial model(s). The 3 initial models evaluated were a multivariable model that incorporated percentage of VLBW cases, percentage of major surgery cases, cardiac surgery as a binary variable (“yes” if ≥5 cases), and ECMO as a binary variable (“yes” if any cases) as separate variables; a single predictor model with NCC; and a single predictor model with CMI. These 3 starting models were compared to one another using Akaike and Bayesian information criteria. Other candidate predictors (region and percentage of inborn cases) were added to the initial models as a “secondary set,” and the effects of their inclusion on model fit were evaluated using likelihood ratio tests. Model-based variance estimators were used for the primary analysis, but final models were assessed via 1000-fold bootstrap resampling to evaluate the influence of resampling on variance estimates and avoid overfitting.
Reported P values represent 2-sided hypothesis tests with P < .05 considered statistically significant. The analysis was performed using Stata version 14 (StataCorp, College Station, TX).
RESULTS
From 152 CDB-RM-participating hospitals with neonatal discharges in 2016, 29 hospitals were excluded due to incomplete data and 5 due to fewer than 100 cases, leaving 118 study hospitals. The volume of neonatal cases per hospital ranged from 116 to 8233 (median, 1367; interquartile range, 676–2104). Across all 118 hospitals, 184 716 cases and 1 561 635 patient-days were represented.
Six hospitals (5%) reported having a level I NICU, 5 (4%) reported level II, and 70 (59%) reported level III; NICU level was not reported for 37 (31%) hospitals. Table 1 shows hospital and clinical population characteristics stratified by derived NCC category. As NCC increased, the median hospital-level average LOS, CMI, and mortality increased and the percentage of inborn cases decreased. The distribution of hospitals by region was similar across NCC.
Table 1.
Hospital and Clinical Population Characteristics by Neonatal Care Complexity Category
| Characteristic | Hospitals (N = 118) | ||
|---|---|---|---|
| Low Complexitya (N = 22) | Medium Complexitya (N = 56) | High Complexitya (N = 40) | |
| Region (N, %) | |||
| Northeast | 6 (27) | 13 (23) | 7 (18) |
| Midwest | 9 (41) | 18 (32) | 11 (28) |
| South | 5 (23) | 14 (25) | 16 (40) |
| West | 2 (9) | 11 (20) | 6 (15) |
| Neonatal case volumeb (median, IQR) | 231 (165–403) | 1441 (974–2289) | 1588 (1327–2524) |
| Length of stay (median, IQR) | 2.6 (2.2–3.3) | 7.5 (5.3–9.0) | 11.6 (9.5–14.1) |
| Case mix index (median, IQR) | 1.72 (1.62–1.84) | 2.11 (2.00–2.32) | 2.51 (2.33–2.68) |
| Percentage of cases with diagnosis/characteristic (median, IQR) | |||
| Inborn admission source | 98 (97–99) | 95 (91–97) | 82 (73–86) |
| Birth weight, g | |||
| <1000 | 0 (0–0) | 1.7 (0.6–2.3) | 2.2 (1.2–3.3) |
| 1000–1499 | 0 (0–0) | 2.6 (1.5–3.9) | 3.6 (2.4–5.1) |
| 1500–2499 | 7.2 (4.7–9.7) | 14.4 (11.8–18.3) | 15.6 (13.0–18.5) |
| Major surgical procedure | 0 (0–0) | 0.6 (0.2–1.4) | 4.1 (3.3–5.3) |
| Cardiac surgical procedurec | 0 (0–0) | 0 (0–0.1) | 0.7 (0.5–1.6) |
| Extracorporeal membrane oxygenation | n/a | n/a | 0.3 (0.1–0.6) |
| Bacterial sepsis | 1.3 (0.3–2.3) | 1.8 (1.0–4.0) | 4.4 (2.9–7.2) |
| Necrotizing enterocolitis | 0 (0–0) | 0.3 (0.2–0.6) | 0.9 (0.6–1.5) |
| Mortality | 0 (0–0.28) | 0.54 (0.31–0.88) | 1.62 (1.17–2.24) |
Abbreviations: IQR, interquartile range; n/a, not applicable.
aNeonatal care complexity hospital level—low: <5 cases with very low-birth-weight (VLBW), < 5 cases with major surgical procedure(s), <5 cardiac surgery cases, and no extracorporeal membrane oxygenation (ECMO) cases; medium: ≥5 VLBW cases or ≥5 cases with major surgical procedure(s), <5 cardiac surgery cases, no ECMO cases; high: ≥5 VLBW cases and either ≥1 ECMO case or ≥5 cardiac surgery cases.
bCount of cases excluding “normal newborn” and nonviable neonates.
cCardiac procedure case percentages based on All Patients Refined Diagnosis Group (APR-DRG) applies only to those with birth weight >2499 g (no applicable APR-DRG for lower-birth-weight neonates).
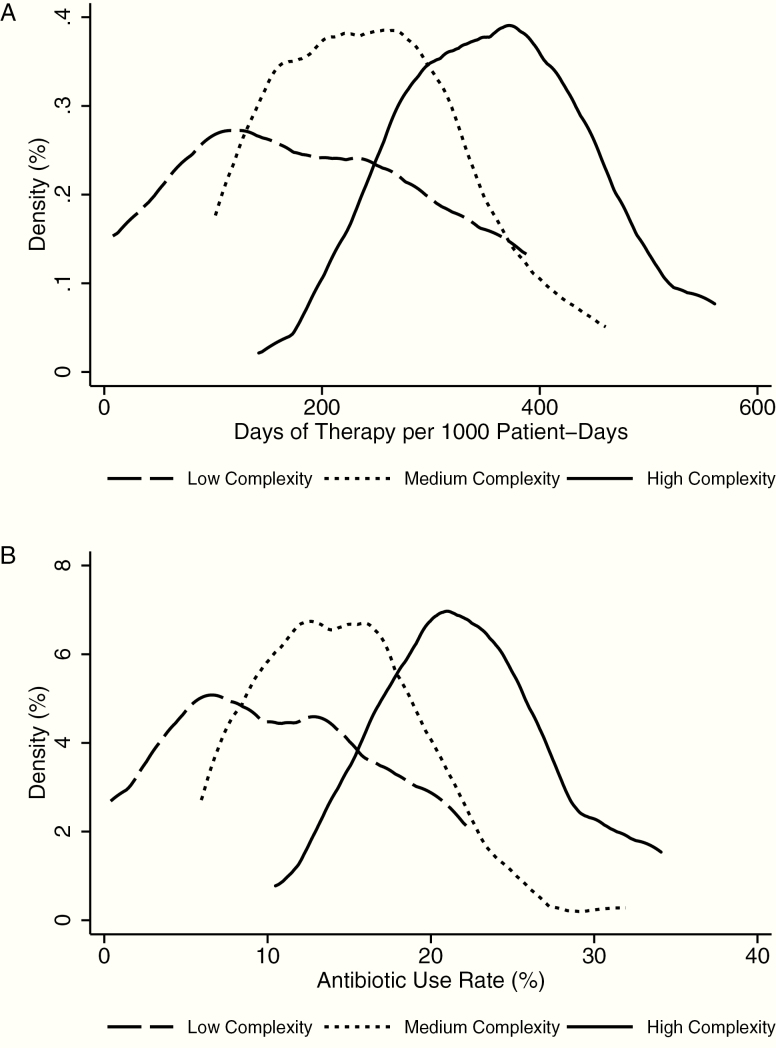
Antibiotic DOT/1000 pd ranged from 7.9 to 561, with a mean of 273 (standard deviation, 118). When stratified by NCC, there were statistically significant differences across all NCC levels in the antibiotic DOT/1000 pd, percentage of cases receiving antibiotics, mean antibiotic days per case, and AUR (antibiotic days as a percentage of days present; Table 2). However, there remained a wide range of antibiotic DOT/1000 pd within NCC categories, with a range of 7.9 to 388 DOT/1000 pd (49-fold difference) in low NCC hospitals, 102 to 461 (4.5-fold difference) in medium NCC hospitals, and 142 to 561 (3.9-fold difference) in high NCC hospitals. The ASI per AD (measuring spectrum of activity) varied less across NCC and between hospitals, with a range of 2 to 7.4. There was a statistically significant difference in mean ASI per AD between low vs high NCC hospitals. Figure 1 shows density distributions of antibiotic DOT/1000 pd and AUR by NCC.
Table 2.
Hospital-level Antibiotic Use Metrics by Neonatal Care Complexity Category
| Antibiotic Use Metric | Hospitals (N = 118) | Analysis of Variance | Pairwise Comparisonsb | ||||
|---|---|---|---|---|---|---|---|
| Low Complexitya (N = 22) Mean (SD) | Medium Complexitya (N = 56) Mean (SD) | High Complexitya (N = 40) Mean (SD) | |||||
| P | P Medium vs Low | P High vs Medium | P High vs Low | ||||
| Metrics for all antibiotic use | |||||||
| DOT/1000 pd | 184 (122) | 243 (88) | 363 (94) | <.001 | .045 | <.001 | <.001 |
| Percent receiving antibiotic | 12 (7) | 24 (14) | 39 (15) | <.001 | .001 | <.001 | <.001 |
| Mean AD/Case | 2.6 (0.6) | 4.5 (1.2) | 7.0 (1.6) | <.001 | <.001 | <.001 | <.001 |
| Antibiotic use rate (%) | 10 (7) | 14 (5) | 22 (6) | <.001 | .013 | <.001 | <.001 |
| Antibiotic spectrum indexc per AD | 5.7 (1.3) | 6.1 (0.6) | 6.4 (0.6) | .007 | .088 | .288 | .005 |
| DOT/1000 pd for individual antibiotics | |||||||
| Ampicillin | 97 (67) | 100 (37) | 108 (30) | .51 | … | … | … |
| Gentamicin | 75 (58) | 76 (34) | 81 (28) | .75 | … | … | … |
| Vancomycin | 0.4 (1) | 17 (18) | 40 (25) | <.001 | .003 | <.001 | <.001 |
| Cefotaxime | 7 (18) | 13 (13) | 22 (17) | .001 | .273 | .019 | .001 |
| Piperacillin-tazobactam | n/ad | 6 (7) | 17 (13) | <.001 | .038 | <.001 | <.001 |
| Amoxicillin | 3 (6) | 3 (4) | 15 (12) | <.001 | >.99 | <.001 | <.001 |
| Cefazolin | 0.2 (1) | 2 (3) | 12 (8) | <.001 | .313 | <.001 | <.001 |
| Cefepime | n/ad | 4 (8) | 9 (7) | <.001 | .086 | <.001 | <.001 |
Abbreviations: AD, antibiotic days; DOT/1000 pd; days of therapy per 1000 patient-days; n/a, not applicable; SD, standard deviation.
aNeonatal care complexity hospital level—low: <5 cases with very low birth weight (VLBW), < 5 cases with major surgical procedure(s), <5 cardiac surgery cases, and no extracorporeal membrane oxygenation (ECMO) cases; medium: ≥5 VLBW cases or ≥5 cases with major surgical procedure(s), <5 cardiac surgery cases, no ECMO cases; high: ≥VLBW cases and either ≥1 ECMO case or ≥5 cardiac surgery cases.
bPairwise comparisons by Tukey test.
cPer Gerber et al [23].
dMedication was not used in any hospital in the low complexity category.
Figure 1.
Density distribution of hospital-level antibiotic days of therapy per 1000 patient-days (A) and antibiotic use rate (B), the percentage of patient-days on which any antibiotic was given, in hospitals with low, medium, and high complexity.
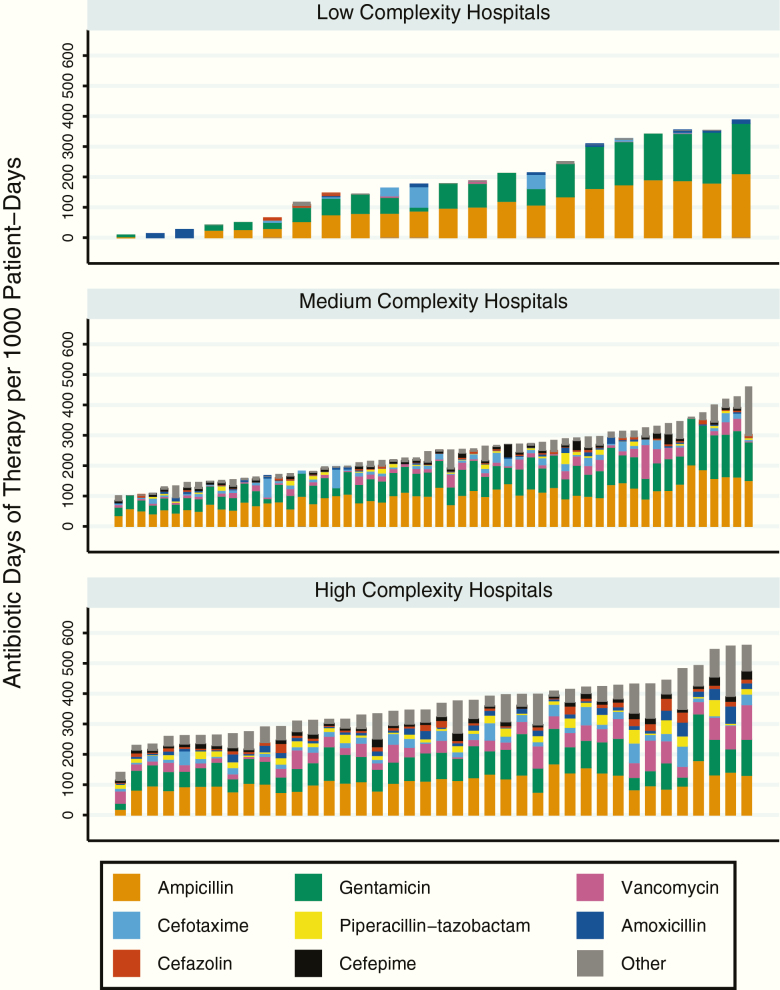
The most frequently used antibiotics by percentage of total DOT in the entire cohort were ampicillin (33% of total DOT) and gentamicin (26% of total DOT), followed by vancomycin, cefotaxime, piperacillin-tazobactam, amoxicillin, cefazolin, and cefepime. These 8 agents accounted for 85% of DOT in the entire cohort. Enterally administered antibiotics accounted for 7% of DOT in the entire cohort. Stratifying the cohort by hospital NCC, ampicillin and gentamicin together comprised 94% of total DOT in low NCC hospitals, 68% of total DOT in medium NCC hospitals, and 53% of total DOT in high NCC hospitals. There were no statistically significant differences in mean DOT/1000 pd for ampicillin or gentamicin across NCC categories. There were statistically significant differences in utilization of other agents (Table 2), with higher utilization of broad-spectrum agents such as vancomycin in medium and high NCC hospitals. Figure 2 shows DOT/1000 pd for individual agents as components of total antibiotic DOT/1000 pd per hospital.
Figure 2.
Antibiotic utilization by complexity and medication. Each bar represents a single hospital. The most frequently used antibiotics are labeled by color, with bar heights adding up to the total antibiotic use in days of therapy per 1000 patient-days for each hospital.
Initial single predictor models (Table 3) identified associations between antibiotic DOT and NCC category, CMI, region, percentage of VLBW cases, percentage of major procedure cases, performance of cardiac surgery, performance of ECMO, and percentage of inborn cases. Neonatal case volume was not associated with DOT. When 3 initial candidate models were compared (CMI alone vs NCC alone vs separate predictors for each component of NCC category), the models with CMI alone and NCC alone showed better fit (Supplementary Table 1). The model with separate predictors showed instability of coefficients and poorer fit, likely due to collinearity of predictors (Supplementary Table 2), and was not considered further.
Table 3.
Single Predictor Relationships Between Hospital and Clinical Population Characteristics and Antibiotic Days of Therapy
| Predictor | Single Predictor Negative Binomial Models | |
|---|---|---|
| Incidence Rate Ratioa (95% Confidence Interval) | P (Wald Test) | |
| Neonatal care complexityb category | ||
| Low | Ref | … |
| Medium | 1.31 (1.05–1.64) | .02 |
| High | 1.95 (1.55–2.47) | <.001 |
| Case mix index | 1.86 (1.50–2.31) | <.001 |
| Percent very low-birth-weight casesc | 1.04 (1.02–1.07) | .001 |
| Percent major procedure cases | 1.07 (1.04–1.10) | <.001 |
| Cardiac surgery performed (≥5 cases) | 1.49 (1.23–1.81) | <.001 |
| Extracorporeal membrane oxygenation performed (any cases) | 1.63 (1.36–1.95) | <.001 |
| Region | ||
| Northeast | Ref | … |
| Midwest | 1.12 (0.87–1.43) | .38 |
| South | 1.34 (1.04–1.73) | .02 |
| West | 1.27 (0.94–1.70) | .12 |
| Percent cases inborn | 0.99 (0.98–0.99) | <.001 |
| Neonatal case volumed | 1.00 (0.99–1.00) | .17 |
a Neonatal care complexity hospital level—low: <5 cases with very low birth weight (VLBW), < 5 cases with major surgical procedure(s), <5 cardiac surgery cases, and no extracorporeal membrane oxygenation (ECMO) cases; medium: ≥5 VLBW cases or ≥5 cases with major surgical procedure(s), <5 cardiac surgery cases, no ECMO cases; high: ≥5 VLBW cases and either ≥1 ECMO case or ≥5 cardiac surgery cases.
b Interpreted as relative increase in days of therapy count, holding patient-days constant, per single unit increase in value of predictor.
c Birth weight <1500 g.
d Count of cases excluding “normal newborn” and nonviable neonates.
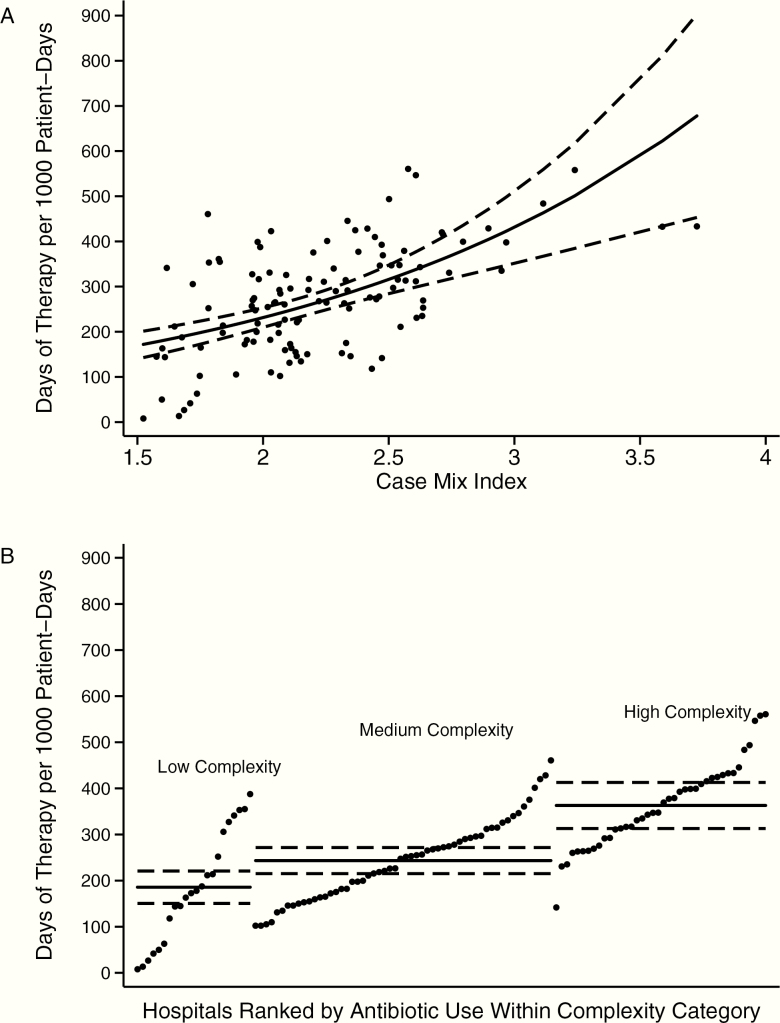
Inclusion of inborn cases and region in the 2 remaining candidate models (CMI vs NCC) did not improve performance of either model based on likelihood ratio tests (Supplementary Table 3), so these predictors were not included in the final models. The model with CMI had minimal changes in confidence intervals with bootstrap resampling (Supplementary Table 4), whereas the model with NCC had wider confidence intervals with resampling but still with statistically significant likelihood ratio test for inclusion of the NCC variable (P < .001). Figure 3 shows actual vs predicted values for the relationships between CMI and antibiotic DOT and between NCC and antibiotic DOT.
Figure 3.
Relationships between hospital-level neonatal antibiotic days of therapy per 1000 patient-days (DOT/1000 pd) and case mix index (CMI) (A) and between DOT/1000 pd and complexity (B). Solid lines represent predicted values, dashed lines represent 95% confidence intervals for predicted values. Dots represent individual hospital’s actual values of DOT/1000 pd plotted against CMI (A) or complexity (neonatal care complexity) (B) (ranked by DOT/1000 pd within category).
DISCUSSION
In this retrospective cohort study of predominantly academically affiliated hospitals in the United States, we found a positive association between hospital-level neonatal antibiotic use, measured in DOT/1000 pd, and complexity of care as indicated by both NCC derived from clinical population characteristics and CMI. We used NCC and CMI to develop 2 candidate models to characterize the relationship between hospital-level antibiotic use and complexity of care. Even after controlling for complexity, there was high interhospital variation in neonatal antibiotic use. The main strengths of our approach are use of a US national dataset that includes validated medication data and measurement of antibiotic use in DOT/1000 pd, which has been infrequently used in neonatal antibiotic use literature [15, 16]. Measurement in DOT is important because it is the recommended antibiotic use metric for antibiotic stewardship programs, it captures combination therapy (more than 1 antibiotic per day), and it measures antibiotic use by individual drugs and clinically important drug classes (eg, broad-spectrum agents) in addition to overall antibiotic use [12].
In contrast to studies by Schulman et al who described hospital-level AUR in California NICUs, we found AUR (and DOT/1000 pd) to be approximately normally distributed vs positively skewed in the California NICU studies [9, 10]. We identified a relationship between antibiotic use and complexity of care, whereas Schulman et al showed similar density distributions of AUR in intermediate NICUs (equivalent to AAP level II, or low NCC in our study), community NICUs (AAP level III, medium NCC), and regional NICUs (AAP level IV, high NCC) in 2016 [10]. In our study, low and medium NCC hospitals had lower AUR in 2016 than reported from intermediate and community NICUs in California in the same year. These differences may be explained, in part, by different measurements. We captured all systemically administered antibiotics (enteral, intravenous [IV], intramuscular [IM]), whereas the California NICU studies included selected IV antibiotics and antifungals in the AUR calculations. Our measurements of antibiotic use were based on validated medication claims data, whereas the California NICU studies were based on hospitals’ self-reported AUR. One important similarity between our findings and those of Schulman et al is the high variation in AUR (and DOT/1000 pd) across hospitals, even when stratifying by complexity.
Flannery et al also reported high variation in the proportion of VLBW and extremely LBW infants who received antibiotics within the first 3 days of life and who received antibiotics for more than 5 days. However, the authors did not specifically address center complexity as a contributor to variation in antibiotic use [11]. O’Leary et al recently reported neonatal antimicrobial use data (antibacterial and antifungal agents, including IV, IM, enteral, and respiratory routes of administration) gathered from hospitals that were participating in the NHSN AU module in 2017 [15]. The highest level of antimicrobial DOT/1000 days present was measured in level III NICUs, followed by special care nurseries, then level II and III NICUs and well newborn nurseries. Relationships between antimicrobial use and NICU level of care or other unit- or facility-specific predictors were not formally tested; however, the findings are qualitatively similar to ours in that the highest complexity unit type showed the highest rate of antimicrobial use.
Evaluation of neonatal antibiotic use in DOT/1000 pd identified other key findings with implications for neonatal antibiotic stewardship. Relatively few antibiotic agents are used in neonates, with the 8 most frequently used drugs accounting for 85% of DOT in the entire cohort. Though ampicillin and gentamicin, which are commonly used for empiric treatment of early-onset sepsis (EOS), were used most frequently, their use did not vary substantially based on hospital NCC category. These findings are similar to those of O’Leary et al from the NHSN AU data [15]. This finding may reflect better standardization of EOS treatment in recent years, with organized stewardship efforts focusing on EOS [29, 30]. Differences in antibiotic DOT/1000 pd across NCC category were driven by higher utilization of antibiotics that may be used to treat hospital-onset conditions (vancomycin, piperacillin-tazobactam, cefepime) or for surgical prophylaxis (cefazolin). This suggests further opportunity for antibiotic stewardship to address antibiotic treatment indications other than EOS, such as late-onset sepsis, necrotizing enterocolitis, and surgical prophylaxis.
We identified 2 main candidate models to characterize the relationship between hospital-level complexity indicators and antibiotic use. The best-performing model used CMI as a single predictor; the other model with acceptable performance used our post hoc NCC category as a single categorical predictor. Before application to clinical practice, each of these models requires further validation for predictive performance. With each of these models, there remains unexplained variation between actual and predicted values of antibiotic DOT/1000 pd. Toward the ultimate goal of understanding what is a reasonable “expected value” of neonatal antibiotic use in a hospital, both CMI and NCC have potential caveats as predictors. Though CMI has been used to risk-adjust antibiotic use in adult inpatients, it was primarily developed as an indicator of predicted resource utilization and may not be an ideal predictor of expected antibiotic use [31]. It is subject to measurement differences based on documentation and coding. Also, although it has been used to some extent in neonatal risk-adjustment for other quality indicators, its calculation is based on weighting of MS-DRGs, which are less granular for neonatal hospitalizations than APR-DRGs [32, 33]. CMI can be applied to claims-based data but cannot be readily applied to data submitted from a hospital unit, such as in the NHSN AU module. For this purpose, prediction models that incorporate unit type (similar to our NCC category) would be more applicable and, based on our findings, could explain some of the variation in neonatal antibiotic use but would still leave substantial unexplained variation. Unexplained variation could represent opportunities for antibiotic stewardship (to reduce avoidable antibiotic use) but also need for better risk-adjustment strategies beyond the hospital-level model, potentially incorporating patient-level risk factors.
Our study has several limitations. Using claims-based data, we were unable to categorize neonatal hospitalizations based on exact location of care; neonates may have been admitted to NICUs, pediatric or cardiac intensive care units, or hospital wards. However, we believe the clinical population that was captured closely approximates patients who would receive care in NICUs. In the majority of study hospitals, most neonates were inborn, and in each NCC group, the median LOS was relatively short. Our post hoc complexity categories were intended to approximate the AAP levels of neonatal care, but it is possible that some hospitals were misclassified [26]. Criteria used in these categories were based on discharge APR-DRGs, which may be subject to misclassification based on documentation and coding. However, based on longer LOS and higher mortality from low to medium to high NCC hospitals, we believe that NCC categories are reasonable proxies for escalating levels of care complexity. Furthermore, misclassification, due either to documentation and coding or flaws in our classification system, is more likely to bias results toward the null hypothesis of no difference between groups. For these reasons, we believe that the main qualitative finding of higher antibiotic use with increasing complexity of neonatal care remains valid.
Because we evaluated aggregate antibiotic use for the entire population of neonates in each hospital and included data aggregated over the entire hospitalization, we could not evaluate the true burden of infection occurrence (eg, if multiple episodes occurred), the linkage between use of antibiotics and specific indications, or the appropriateness of antibiotic therapy.
Regarding generalizability to other settings, the Vizient CDB-RM includes primarily academically affiliated hospitals and may not accurately represent neonatal antibiotic use in community hospitals. Participating hospitals are primarily hospitals with newborn deliveries (only 2 of 118 hospitals had no inborn neonates). Antibiotic use may vary in hospitals that care only for outborn neonates (such as independently licensed children’s hospitals), and further study of neonatal antibiotic use in such hospitals is warranted. Actual numbers for DOT/1000 pd may differ from DOT per 1000 days present, the metric used by the NHSN AU module [14, 15]. Validation of our findings in a variety of neonatal care settings and identification of other risk modifiers are needed for widespread applicability.
CONCLUSIONS
We have shown that neonatal antibiotic use varies based on complexity of neonatal care, with increasing antibiotic use from low to medium to high complexity hospitals. Models based on NCC and CMI show promise for risk-adjusted interhospital comparison. Even with adjustment for complexity, neonatal antibiotic use varies substantially between hospitals. Considering the major health risks of early life antibiotic exposure, it is important to develop and validate risk adjustment methods to accurately identify expected hospital-level antibiotic use and direct antibiotic stewardship efforts for neonates at every level of care.
Supplementary Material
Notes
Acknowledgments. The authors thank Dr Ward Hagar, University of California San Francisco Benioff Children’s Hospital Oakland, for critical review of the study concept and Dr Andrew D. Auerbach, University of California San Francisco, for critical review of the manuscript.
Disclaimer. Data from the Vizient Clinical Database/Resource Manager was used by permission of Vizient. All rights reserved.
Financial support. This work was supported by post-doctoral fellowship support provided to P. S. by the University of California San Francisco Benioff Children’s Hospital Oakland.
Potential conflicts of interest. All authors: No reported conflicts of interest. The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Grohskopf LA, Huskins WC, Sinkowitz-Cochran RL, Levine GL, Goldmann DA, Jarvis WR. Use of antimicrobial agents in United States neonatal and pediatric intensive care patients. Pediatr Infect Dis J 2005; 24:766–73. [DOI] [PubMed] [Google Scholar]
- 2. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics 2006; 117:1979–87. [DOI] [PubMed] [Google Scholar]
- 3. Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics 2006; 118:717–22. [DOI] [PubMed] [Google Scholar]
- 4. Ting JY, Synnes A, Roberts A, et al. Association between antibiotic use and neonatal mortality and morbidities in very low-birth-weight infants without culture-proven sepsis or necrotizing enterocolitis. JAMA Pediatr 2016; 170:1181–7. [DOI] [PubMed] [Google Scholar]
- 5. Cantey JB, Huffman LW, Subramanian A, et al. Antibiotic exposure and risk for death or bronchopulmonary dysplasia in very low birth weight infants. J Pediatr 2017; 181:289–293. [DOI] [PubMed] [Google Scholar]
- 6. Cantey JB, Pyle AK, Wozniak PS, Hynan LS, Sánchez PJ, et al. Early antibiotic exposure and adverse outcomes in preterm, very low birth weight infants. J Pediatr 2018; 203:62–7. [DOI] [PubMed] [Google Scholar]
- 7. Spitzer AR, Kirkby S, Kornhauser M. Practice variation in suspected neonatal sepsis: a costly problem in neonatal intensive care. J Perinatol 2005; 25:265–9. [DOI] [PubMed] [Google Scholar]
- 8. Yves Liem TB, Krediet TG, Fleer A, Egberts TCG, Rademaker CMA. Variation in antibiotic use in neonatal intensive care units in the Netherlands. J Antimicrob Chemother 2010; 65:1270–5. [DOI] [PubMed] [Google Scholar]
- 9. Schulman J, Dimand RJ, Lee HC, Duenas GV, Bennett MV, Gould JB. Neonatal intensive care unit antibiotic use. Pediatrics 2015; 135:826–33. [DOI] [PubMed] [Google Scholar]
- 10. Schulman J, Profit J, Lee HC, et al. Variations in neonatal antibiotic use. Pediatrics 2018; 142:e20180115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flannery DD, Ross RK, Mukhopadhyay S, Tribble AC, Puopolo KM, Gerber JS. Temporal trends and center variation in early antibiotic use among premature infants. JAMA Netw Open 2018; 1:e180164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fridkin SK, Srinivasan A. Implementing a strategy for monitoring inpatient antimicrobial use among hospitals in the United States. Clin Infect Dis 2014; 58:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Santen KL, Edwards JR, Webb AK, et al. The standardized antimicrobial administration ratio: a new metric for measuring and comparing antibiotic use. Clin Infect Dis 2018; 67:179–85. [DOI] [PubMed] [Google Scholar]
- 15. O’Leary EN, van Santen KL, Edwards EM, Braun D, Buus-frankME. Using NHSN’s antimicrobial use option to monitor and improve antibiotic stewardship in neonates. Hosp Pediatr 2019; 9:340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cantey JB, Wozniak PS, Pruszynski JE, Sánchez PJ. Reducing unnecessary antibiotic use in the neonatal intensive care unit (SCOUT): a prospective interrupted time-series study. Lancet Infect Dis 2016; 16:1178–84. [DOI] [PubMed] [Google Scholar]
- 17. Vizient Clinical Database-Resource Manager. Available at: https://www.vizientinc.com. Accessed 9–11 January 2018.
- 18. Vizient, Inc. Available at: https://www.vizientinc.com. Accessed 12 August 2019.
- 19. Gerber JS, Newland JG, Coffin SE, et al. Trends in antibacterial use in US academic health centers: 2002 to 2006. Pediatrics 2010; 168:e1294–300. [Google Scholar]
- 20. Polk RE, Hohmann SF, Medvedev S, Ibrahim O. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis 2011; 53:1100–10. [DOI] [PubMed] [Google Scholar]
- 21. Stultz JS, Kohinke R, Pakyz AL. Variability in antifungal utilization among neonatal, pediatric, and adult inpatients in academic medical centers throughout the United States of America. BMC Infect Dis 2018; 18:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vizient, Inc. Vizient Clinical Data Base / Resource Manager TM User Manual. Irving, TX. Updated, 2017. Available at https://www.vizientinc.com/-/media/documents/sitecorepublishingdocuments/secured/learningcenter/cdb_user_guide.pdf.
- 23. Gerber JS, Hersh AL, Kronman MP, Newland JG, Ross RK, Metjian TA. Development and application of an antibiotic spectrum index for benchmarking antibiotic selection patterns across hospitals. Infect Control Hosp Epidemiol 2017; 38:993–7. [DOI] [PubMed] [Google Scholar]
- 24. Lahart A, McPherson C, Gerber J, Warner B, Lee B, Newland J. Application of an antibiotic spectrum index in the neonatal intensive care unit. Infect Control Hosp Epidemiol 2019; Jul 29:1–3. [DOI] [PubMed] [Google Scholar]
- 25. MS-DRG Classifications and Software. Available at: www.cms.gov. Accessed 8 February 2019.
- 26. American Academy of Pediatrics Committee on Fetus and Newborn. Levels of neonatal care. Pediatrics 2012; 130:587–97. [DOI] [PubMed] [Google Scholar]
- 27. Song AY, Chen HHA, Chapman R, et al. Utilization patterns of extracorporeal membrane oxygenation in neonates in the United States 1997–2012. J Pediatr Surg 2017; 52:1681–87. [DOI] [PubMed] [Google Scholar]
- 28. Bhatt P, Lekshminarayanan A, Donda K, et al. National trends in neonatal extracorporeal membrane oxygenation in the United States. J Perinatol 2018; 38:1106–13. [DOI] [PubMed] [Google Scholar]
- 29. Higgins RD, Saade G, Polin RA, et al. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet Gynecol 2016; 127:426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuzniewicz MW, Puopolo KM, Fischer A, et al. A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr 2017; 171:365–71. [DOI] [PubMed] [Google Scholar]
- 31. Kuster SP, Ruef C, Bollinger AK, et al. Correlation between case mix index and antibiotic use in hospitals. J Antimicrob Chemother 2008; 62:837–42. [DOI] [PubMed] [Google Scholar]
- 32. Smith AH, Gay JC, Patel NR. Trends in resource utilization associated with the inpatient treatment of neonatal congenital heart disease. Congenit Heart Dis 2014; 9:96–105. [DOI] [PubMed] [Google Scholar]
- 33. Muldoon J. Structure and performance of different DRG classification systems for neonatal medicine. Pediatrics 1999; 103:302–18. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.