Highlights
-
•
Phoslock® decreases iron-rich sediment-water P flux when bottom waters are anoxic.
-
•
Phoslock® does not decrease sediment-water P flux when bottom waters are oxic.
-
•
Phoslock® is a source of N—NH4+ to the water column when dispersed in lake water.
Keywords: Phoslock®, Phosphate flux, Nutrient management, Core incubations, Oxia, Anoxia, Iron-rich sediments
Abstract
Eutrophication in lakes and reservoirs has prompted interest in using sediment capping technology to reduce the sediment contribution to internal nutrient loading. One such sediment capping technology is Phoslock®, a lanthanum-embedded clay, which can bind phosphate at the sediment surface and limit its diffusion into the water column. However, in well-oxygenated lakes, naturally occurring iron can bind phosphate by a similar mechanism. We sought to test the efficacy of Phoslock® in limiting phosphate (PO43−) fluxes relative to untreated iron-rich lake sediment under conditions of bottom-water oxia and anoxia through laboratory batch core incubations of intact sediment cores from Jordan Lake, a reservoir in central North Carolina. We found that Phoslock® decreased phosphate fluxes relative to the control under anoxic conditions (7.5 ± 9.5 vs. 236 ± 74 µmol PO43−•m−2•d−1), but provided no benefit relative to the control when the water column was oxygenated (4.5 ± 4.3 vs. 7.0 ± 11.4 µmol PO43−•m−2•d−1). We also found that Phoslock® itself can act as a source of NH4+ to Jordan Lake waters. Applied at recommended levels to the whole lake, Phoslock® addition would result in a pulse increase in water column NH4+ concentrations of approximately 2.6 ± 0.8 μM (an increase of 10 to 275% compared to ambient).
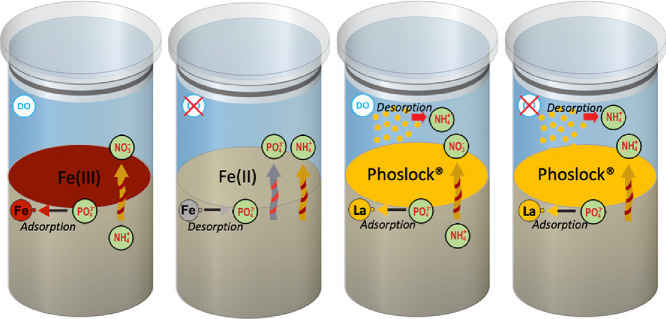
Graphic Abstract
1. Introduction
Nutrient loading, including both nitrogen (N) and phosphorus (P), is responsible for widespread and long-term eutrophication in lakes, a process that is increasing in magnitude as humans continue to affect freshwater ecosystems through agriculture and development (Dodds et al., 2013; Smith et al., 2006; Wurtsbaugh et al., 2019). Once nutrients enter a freshwater lake or reservoir from the watershed, they can stimulate primary productivity and harmful algal blooms, and enhance the delivery of organic matter to the sediments (Le Moal et al., 2019). Here, various remineralization processes regenerate nutrients that accumulate in the porewater and can be returned to the water column through processes of diffusion (Berg et al., 1998), bioirrigation (Murniati et al., 2017; Renz et al., 2018), and bioturbation (Berg et al., 2001). As a result of this benthic recycling (often referred to as ‘internal nutrient loading’), nutrients that enter a lake can contribute to eutrophication for many years (Søndergaard et al., 2013).
Strategies for coping with nitrogen loading often rely on the establishment of denitrification-favorable conditions (Vymazal, 2007), aimed at permanent removal of reactive N from the environment by the biological reduction to non-reactive N2 gas. However, managers hoping to reduce or reverse eutrophication in lakes often focus on reducing phosphorus loading (Schindler et al., 2008, 2016), which increases the N:P ratio, potentially limiting the dominance of N2-fixing cyanobacteria that can be a health hazard and nuisance (Schindler, 2012). Still, the concept that P-loading alone can solve lake eutrophication is controversial (Paerl et al., 2011), due to the negative impacts that excess N can have on lake macrophyte communities (Moss et al., 2013). Additionally, managed reductions of P in lakes without accompanying N management can decrease the ability of lakes to remove reactive N through nitrification/denitrification (Finlay et al., 2013), and exacerbate downstream eutrophication in often N-limited estuaries and coastal areas (Paerl, 2009).
In some lakes, especially those with clay-rich sediments, iron can effectively ‘cap’ the release of phosphate from the sediments when the water column is oxygenated, through the redox-dependent formation of Fe(III)-phosphate complexes in the oxygenated layers of sediment (Orihel et al., 2015, 2016). Additionally, lake sediments with low sulfide and high iron can permanently sequester P, through the formation of the stable mineral vivianite in anoxic sediments, eventually reducing concentrations of leachable P and thus its release to the water column (Gächter and Müller, 2003; Rothe et al., 2014). When these natural processes are insufficient sinks for P loading, and/or it is not practical to decrease P loading from the watershed, stakeholders may increasingly turn to engineering solutions, such as sediment capping or dredging, to decrease internal P loading (Lürling et al., 2017; Lürling and Faassen, 2012; Guido Waajen et al., 2016; Zamparas and Zacharias, 2014). One such approach is the capping of sediment with lanthanum-embedded bentonite clay, known commercially as Phoslock®.
Phoslock® has already been applied to a variety of lakes worldwide, across broad ranges in morphology and designated use (Bishop et al., 2014; Bishop and Richardson, 2018; Copetti et al., 2015; Dithmer et al., 2016b; Spears et al., 2013, 2016). While these studies generally show a reduction in water column soluble reactive phosphorus (SRP), other studies have highlighted unintended impacts of Phoslock® on sediment biogeochemistry, such as changing the location of the sediment oxic-anoxic boundary (Vopel et al., 2008). Additional risks include ecological toxicity, related to the leaching of lanthanum from Phoslock®, especially in lakes of low alkalinity (Gibbs et al., 2011; Reitzel et al., 2017; Spears et al., 2013), and associated bioaccumulation of lanthanum in the ecosystem has been reported (Van Oosterhout et al., 2014; G. Waajen et al., 2017).
Other studies have indicated that Phoslock® may act as a direct source of ammonium (NH4+) when leached with ultrapure water in the laboratory, including nanopore (van Oosterhout and Lürling, 2013) and Milli-Q (Reitzel et al., 2013) water. This ammonium may come from the bentonite clay matrix itself (Hanway et al., 1957) and may not be removed during the Phoslock® manufacturing process. While studies have shown that the Phoslock® clay matrix can be unstable under particular natural lake conditions, such as low alkalinity and/or high humic substance content, as demonstrated by the release of La (Reitzel et al., 2017; Spears et al., 2013), studies have yet to demonstrate whether NH4+ is leached from Phoslock® in natural lake water. Furthermore, evidence is presently lacking for the efficacy of Phoslock® on intact sediment-water interfaces, as opposed to homogenized surface sediment (Egemose et al., 2010; Gibbs et al., 2011; Reitzel et al., 2013; Wang et al., 2016), where its effects on sediment biogeochemistry are expected to be more representative of the natural lake bed. Core incubation studies have demonstrated that Phoslock® amendment can decrease the release of P from homogenized sediments from Lake Rotorua (Gibbs et al., 2011) and Lake Langesø (Reitzel et al., 2013) relative to untreated sediment, even under oxic conditions when surface iron(III) oxides would be potentially available to bind P. It is probable that the relative efficacy of Phoslock® is dependent on characteristics of particular lakes, such as the iron content in the sediments, and we were interested in testing this relative efficacy for clay-rich lake sediments common to the southeastern United States.
In this study, we tested the relative efficacy of Phoslock® in reducing benthic phosphate fluxes compared to untreated iron-rich cores under conditions of bottom-water oxia and anoxia. This study took place in a shallow reservoir in central North Carolina, representative of Piedmont, clay-rich reservoirs throughout the southeastern United States. We used batch incubations of intact sediment cores to measure the impact of Phoslock® on sediment-water phosphate fluxes under conditions of oxia and anoxia, and also observed whether ammonium is leached from Phoslock® with natural lake water.
2. Methods
2.1. Study site
B. Everett Jordan Lake (Fig. 1) is a reservoir in central North Carolina that provides drinking water for the towns of Cary, Apex and Morrisville, and Chatham and Wake Counties, as well as recreation and other services to the broader Triangle region (Berke et al., 2013). The reservoir was constructed from 1973 to 1983 with the damming of the Haw and New Hope rivers, within the Cape Fear River drainage basin. Jordan Lake has a long history of impaired water quality, frequently failing to meet United States Environmental Protection Agency (US EPA) guidelines for Chlorophyll a, along with year-round detectable concentrations of microcystin and anatoxin (Wiltsie et al., 2018). While Jordan Lake is shallow (average depth 4.3 m, max 12 m) and generally well-mixed, some sections experience stratification and bottom-water anoxia during the summer (Cain, 2017).
Fig. 1.
Map of our site (pink star) within Jordan Lake, and within the eastern United States (inset). All three sampling campaigns were conducted in the same area of Jordan Lake.
2.2. Sampling strategy
Short sediment cores (~15–30 cm in length, with core length determined by the availability of sediment above the historic riverbed) were collected using a Kajak-Brinkhurst (KB) corer with 4.7 cm (id) core liner (Wildco Supply Company). All cores were taken near the North Carolina Division of Water Resources station CPF086F, northeast of the State Road 1008 bridge in Jordan Lake (Fig. 1), between October 2017 and April 2018. In October 2017, 8 cores were collected, with 4 designated for use in the flux experiments and 4 sectioned immediately for pore-water and solid-phase analysis. In February 2018, 8 cores were again collected (5 for flux experiments and 3 for immediate sectioning), while in April 2018, 7 cores were collected (5 for flux experiments and 2 for immediate sectioning). Cores were collected during a ~1–2 h period, capped immediately along with the overlying water, and stored upright on ice during transport within ~1 h to a refrigerated room (4 °C) where they were kept in the dark until either sectioning or flux experiments.
In addition to the cores, 20 L of bottom water was collected during each sampling trip from 0.5 m above the sediment surface with a Van Dorn sampler and stored in 10-L acid washed Cubitainers®. This bottom water was quickly (~1 h) returned to a refrigerated room, where it was kept until use in the flux experiments. Bottom water (10 mL) was subsampled, filtered (0.45 µm), and frozen for phosphate (PO43−), ammonium (NH4+), and nitrate + nitrite (NO3−+ NO2−) concentration analysis at Wetland Biogeochemistry Analytical Services (WBAS, Louisiana State University). Standard US EPA methods were applied for all nutrient analyses (O'Dell, 1993; Zhang et al., 1997; Zimmermann and Keefe, 1997).
2.3. Flux experiments
Flux experiments began within 2 weeks of sediment core collection, and 3 separate experiments were conducted to test the impact of Phoslock® and redox conditions on sediment-water nutrient fluxes. Experiment 1, with the first collection of cores, tested nutrient fluxes in unaltered sediment under conditions of bottom water oxia and anoxia. Experiment 2, with the second collection of cores, considered the effect of Phoslock treatment with oxic bottom waters. Experiment 3, with the third collection of cores, considered the effect of Phoslock treatment with anoxic bottom waters. The use of unaltered sediment as controls in experiments 2 and 3, allowed us to test the relevant research question while minimizing the influence of seasonal differences between late winter and early spring. For each experiment, fluxes were generally measured multiple times per core after the replacement of overlying water with fresh bottom water, and these separate flux measurements are referred to as ‘trials’. With the combination of replicate cores and trials, multiple pseudo-replicates were established for each treatment condition.
Prior to the first trial of each experiment, cores and bottom water were allowed to equilibrate to room temperature (19–20 °C), and overlying water was carefully removed from each core via syphon and replaced with 150 mL of bottom water. This volume was chosen to be small enough to be responsive to benthic nutrient fluxes, yet large enough to not be substantially affected by the removal of 9–10 mL sample volume. Deviations from the following procedure when Phoslock® was dosed are described in the next paragraph. Humidified air or nitrogen gas (depending on oxic vs. anoxic treatment) was bubbled through the overlying water at a metered flow rate sufficient to ensure mixing of the water column without re-suspending sediment, using J-shaped steel HPLC tubing which lay just above the sediment water interface. In this way, the bubbles flowed gently upwards from the sediment-water interface, mixing the water column without resuspending the sediment. This rate was tested and confirmed with a dye-tracer experiment. All cores were wrapped with aluminum foil throughout the experiment to keep the sediments and overlying water dark, and the temperature of the room, measured multiple times each day, was consistently 19–20 °C. Overlying water samples were taken at known time intervals, with the initial sample taken at the start of gas bubbling, over a period of 1–5 days. Samples were filtered (0.45 µm, 9–10 mL), and frozen prior to analysis for PO43−, NH4+, and NO3− + NO2− at WBAS. After removing an aliquot of overlying water, the same volume of bottom water was added to each core, maintaining an overlying water volume of 150 mL. Nutrient concentrations in this bottom water were measured for each sampling day, and used to correct the overlying water concentration for the effect of replacement. For Experiment 1, all 4 cores were run under oxic conditions for the first trial, after which the bottom water was replaced and 2 cores were run under oxic conditions and 2 cores under anoxic conditions for the second trial.
For Experiments 2 and 3, three cores were treated with Phoslock® and two cores remained untreated as the control, and the experiments continued over the course of 3 trials. Sediment cores treated with Phoslock® were dosed prior to the start of the first trial only. Phoslock® was provided by SePRO Corporation (SePRO Research and Technology Campus, Whitakers North Carolina, USA). The mass of Phoslock® added was determined from the proposed addition to Jordan Lake given by SePRO at 1.8 m3•ha−1 scaled to the surface area of the core (West Bishop, personal communication). This amounted to 0.5 g Phoslock® per 4.7 cm (id) diameter core, creating a Phoslock® layer approximately 1–2 mm thick. This dosage is in the range of dosage rates used in previous studies (e.g., 100–200% the dosage used by Gibbs et al., 2011 and ~25% of the dosage rate of Reitzel et al., 2013). When Phoslock® was dosed, 140 mL of bottom water was added to the core instead of the full 150 mL. Then, 5 mL overlying water was added to a scintillation vial containing 0.5 g Phoslock®, which was poured over the core, followed by 5 mL of overlying water as a rinse. Our application method was intended to create an even distribution of Phoslock® across the sediment-water interface. After Phoslock® had settled (5–6 h), gas flow was turned on for all Phoslock® and control cores, and the initial sample of the experiment was taken.
The PO43− flux was calculated from the slope of corrected PO43− concentration vs. time multiplied by the volume of overlying water and divided by the surface area of the sediment. In the first trial of anoxic treatments (Experiment 1 - trial 2, or Experiment 3 – trial 1), PO43− fluxes appeared to be delayed by ~20 h, which was likely caused by the slow transition of surface sediments from oxic to anoxic. More precisely, we presume this time-lag was caused by the slow reduction of Fe(III) to Fe(II), given our observation of a change in color from red to gray at the sediment surface. This is consistent with recent observations from Lake Erie, where PO43− release was not observed under hypoxic conditions, but instead was observed between 12 and 24 h following the onset of anoxic conditions (Anderson et al., 2021). Due to this suppression of PO43− fluxes at the start of the anoxic trials, PO43− fluxes were only calculated after this 20-hour time window had passed, once the PO43− concentration started increasing. The NH4+ flux was calculated in a similar manner. However, because we observed a non-negligible accumulation of NH4+ in the overlying water at longer incubation times (which artificially depresses measured and calculated sediment-water fluxes), we calculated NH4+ fluxes using the first 24–40 h of each trial.
2.4. Core sectioning and porewater chemistry
Sediment cores selected for immediate sectioning were processed within 1 week of retrieval from Jordan Lake, while those used in flux experiments were sectioned 1–3 days following the termination of the flux experiment. Prior to core sectioning, overlying water was carefully removed via syphon, and cores were extruded at 1-cm intervals for the top 3 cm, followed by 3-cm intervals for the remainder of the core. Extruded sediment was collected in clean 65-mL centrifuge tubes, cooled in a refrigerator (4 °C), then centrifuged at 4 °C to separate porewater and sediment. Porewater was passed through a 0.45-µm syringe filter and frozen until PO43−, NH4+, and NO3− + NO2− analysis at WBAS. As we did not expect to find NO3− + NO2− below 3 cm, we analyzed NO3− + NO2− for the top 3 cm only. Care was taken throughout the process to minimize the introduction of oxygen prior to filtering, which could decrease the amount of PO43− by forming particulate Fe(III) complexes. Samples for PO43− were acidified prior to analysis, which would free any PO43− adsorbed on Fe(III) in the post-filtered sample.
3. Results
3.1. Flux experiments
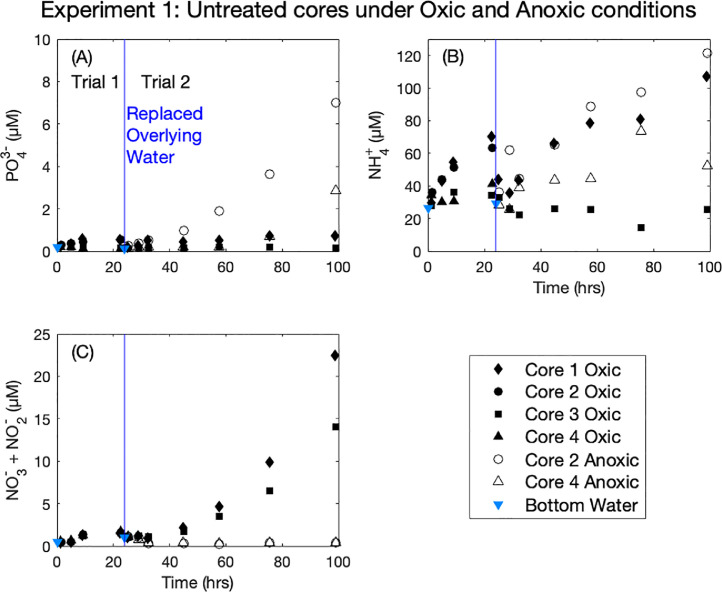
In Experiment 1 (Oxia vs. Anoxia in untreated lake sediment), we found that PO43− did not appreciably increase with time for untreated cores under oxic conditions (Fig. 2, (A), filled symbols) for either trial, however under anoxic conditions there was a measurable release of PO43− from the sediment (Fig. 2, (A), open symbols). We observed a ~20-hour delay of PO43− release for Cores 2 and 4, presumably due to the time required to reduce Fe(III) to Fe(II) on the sediment surface as the cores transitioned from oxic to anoxic conditions (Anderson et al., 2021). We observed variable response of NH4+ under oxic conditions, with concentrations increasing during the first Trial by large amounts for Core 1 and Core 2 and smaller amounts for Core 3 and Core 4. Increases in NH4+ continued for Core 1 in the second trial, while reductions in NH4+ were observed for Core 3 (Fig. 2, (B), filled symbols). Under anoxic conditions, overlying NH4+increased the most for Core 2, with a moderate increase observed for Core 4 (Fig. 2, (B), open symbols). Changes in NO3− + NO2− were not observed under anoxic conditions (Fig. 2, (C), open symbols) while increases were observed under oxic conditions, especially in Trial 2 after ~48 h had passed (Fig. 2, (C), filled symbols).
Fig. 2.
Corrected overlying water nutrient concentrations for Experiment 1 separated by analyte (PO43− (A), NH4+ (B), and NO3− + NO2 (C)). Coloring is consistent for all figures, with black colors denoting untreated control cores. Filled symbols indicate oxic conditions and open symbols indicate anoxic conditions. Each core has a unique symbology, for example Core 2 is a circle under both oxic (filled) and anoxic (open) conditions. Vertical blue lines in all graphs indicate the replacement of bottom water and the start of the subsequent trial. Bottom waters were subsampled for nutrient analysis prior to each replacement of overlaying water (blue triangles).
In Experiment 2 (Phoslock® treatment under oxic conditions), we found that the overlying water PO43− concentration did not appreciably change with time under oxic conditions for both Phoslock® treatment and the control, for all three trials (Fig. 3, (A), note the y-axis scale). For both NH4+ and NO3−+ NO2− we found very strong differences between trials (Fig. 3, (B) and (C)). NH4+ concentrations at the first timepoint were elevated in the Phoslock® treatments, and remained slightly elevated relative to bottom water even after overlying water was replaced for the second trial (Fig. 3, (B), red symbols and blue triangle). Despite the elevated initial concentration, we observed NH4+ uptake in each subsequent trial for both Phoslock® and control, which we attribute to apparent nitrification (Fig. 3, (B)). Bottom waters collected for Experiment 2 were elevated in NO3− + NO2− compared to bottom waters for Experiments 1 and 3 (Fig. 3, (C), blue triangles). This concentration gradient fueled sediment uptake of NO3− and NO2− during the first half of Trial 1 for both Phoslock® and control (Fig. 3, (C)). In subsequent trials, overlying water NO3−+ NO2− concentrations increased with time (Fig. 3, (C)), most strongly for the Phoslock® treated core 4.
Fig. 3.
Corrected overlying water nutrient concentrations for Experiment 2 separated by analyte (PO43− (A), NH4+ (B), and NO3− + NO2 (C)). Coloring is consistent for all figures, with black colors denoting untreated control cores, and red colors denoting Phoslock® treatment. Filled symbols indicate oxic conditions. Vertical pink lines indicate the delay between the first overlaying water replacement and Phoslock® addition, and the start of air bubbling and subsequent first sample, about 5 h. Vertical blue lines indicate the replacement of bottom water and the start of the subsequent trial. Bottom waters were subsampled for nutrient analysis prior to each replacement of overlaying water (blue triangles).
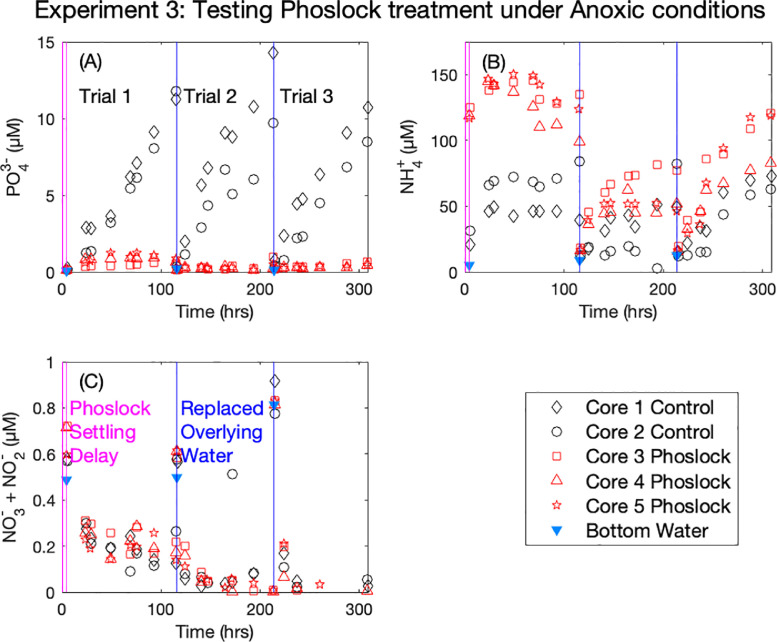
In Experiment 3 (Phoslock® treatment under anoxic conditions), we found that under anoxic conditions untreated sediment cores exhibited a measurable PO43− concentration increase (Fig. 4, (A), black symbols), while Phoslock® treatment prevented such PO43− release under otherwise identical conditions (Fig. 4, (A), red symbols). We again observed an increase in NH4+ concentration for the first timepoint of Trial 1 with Phoslock® treatment (Fig. 4, (B), red symbols). For untreated cores, as well as Phoslock® treated cores after Trial 1, NH4+ concentrations increased in the overlying water with time, although this trend was weaker for Core 2 in Trial 2 (Fig. 4, (B)). NO3− + NO2− concentrations remained low for all treatments and cores, consistent with the suppression of nitrification under anoxia (Fig. 4, (C), note the y-axis scale). We are cautious about interpreting the relative impact of Phoslock® on the nitrogen cycle under either oxic or anoxic conditions, because the potential impact of the large and sudden increase in NH4+ concentration (130–240 µM) is enhanced by our experimental choice of a batch incubation setup. However, previously reported flow through incubations may have missed this NH4+ release due to the exchange of water between Phoslock® dosing and the start of the experiment (Gibbs et al., 2011). Due to our artificially small overlaying water volume (150 mL vs ~7.5 L for the average water column depth over the same area in Jordan Lake), the concentration increases in NH4+ due to Phoslock® treatment would be much greater in our experimental setup than it would if applied to Jordan Lake. This, in turn, could have lingering impacts on NH4+ and NO3−+ NO2− observations in subsequent Trials either by artificially affecting nitrification and subsequent denitrification rates, or by artificially increasing the porewater concentrations in the upper few centimeters of the core.
Fig. 4.
Corrected overlying water nutrient concentrations for Experiment 3 separated by analyte (PO43− (A), NH4+ (B), and NO3− + NO2 (C)). Coloring is consistent for all figures, with black colors denoting untreated control cores, and red colors denoting Phoslock® treatment. Open symbols indicate anoxic conditions. Vertical pink lines indicate the delay between the first overlaying water replacement and Phoslock® addition, and the start of N2 bubbling and subsequent first sample, about 5 h. Vertical blue lines indicate the replacement of bottom water and the start of the subsequent trial. Bottom waters were subsampled for nutrient analysis prior to each replacement of overlaying water (blue triangles).
3.2. Core profiles and bottom water chemistry
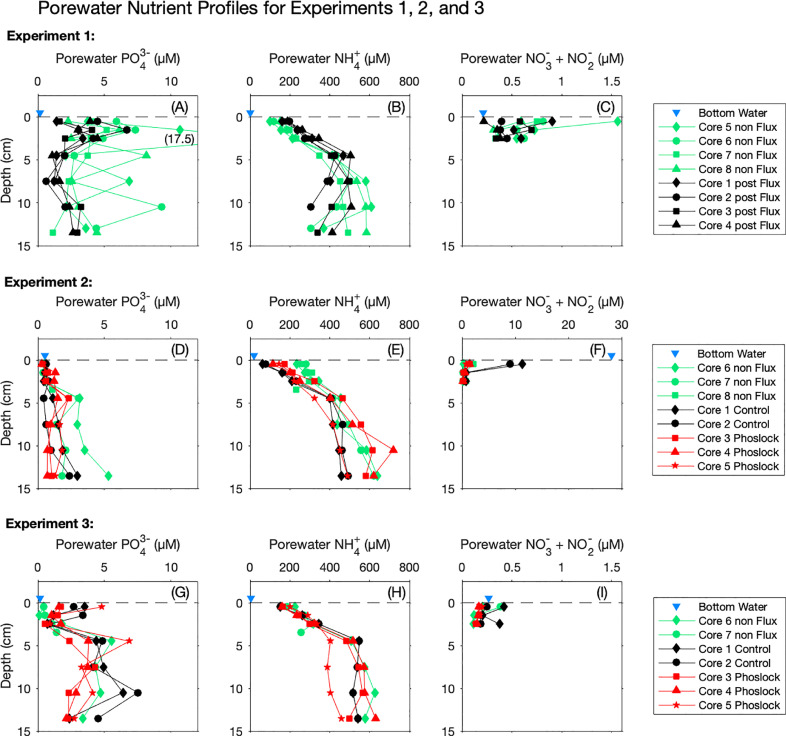
Our approach to quantifying sediment-water fluxes requires that porewater PO43− and NH4+ concentrations remained stable for the 0–2 weeks prior to the start of the flux experiment (storage at 4 °C), and for the 5–15-day duration of the flux experiments (room temperature). In general, porewater nutrient profiles for cores that were sectioned shortly after collection (Fig. 5 all charts, non Flux, green) were similar to profiles of cores that were sectioned after the completion of the flux experiments (Fig. 5 all charts, black and red), however there are some important deviations to point out. For each treatment (non Flux, post Flux, Control, and Phoslock) and experiment, we conducted ANOVA (RStudio) to compare the means. While the small sample size (n = 2–4) is an issue for this analysis, the results for the top 5 cm can be found in the supporting information (Supplementary Figure S1). In Experiment 1, Core 5 non Flux showed elevated PO43− concentrations in the top three cm (Fig. 5, (A), green diamond), although the other three non Flux cores were much more similar to the post Flux cores (Fig. 5, (A)), and the differences were not significant (p > 0.1). In depths lower than the top three cm, Core 8 and Core 6 non Flux also had at least one elevated fraction (Fig. 5, (A), green circle and triangle), however it should be noted that the flux experiments would be most sensitive to the uppermost fraction PO43− concentrations, as these have the greatest influence on diffusive fluxes. Post Flux cores showed a small elevation in NH4+ concentration relative to non Flux cores (p < 0.05) in the uppermost fractions, and this trend was reversed at depths below 7 cm (Fig. 5, (B)). For NO3− + NO2−, all concentrations were low for both post Flux and non Flux cores (Fig. 5, (C)) although a slight elevation was observed at 0.5 cm for Core 5 non Flux (Fig. 5, (C), green diamond). For this experiment, a trend in NH4+ or NO3− + NO2− porewater concentrations based on Trial 2 oxic treatment (Core 1 and Core 3) vs anoxic treatment (Core 2 and Core 4) could not be established.
Fig. 5.
Porewater nutrient profiles of sectioned cores, separated by analyte (PO43−, NH4+, and NO3−+ NO2−) and Experiment (1, 2, and 3). non Flux (Green): Cores which were sectioned upon collection. Control (black): untreated cores which underwent flux incubations prior to sectioning. Phoslock (red): Phoslock® treated cores which underwent flux incubations prior to sectioning. Points are placed at the midpoint depth of each fraction. Many cores were longer than 15 cm, however only the first 15 cm are presented here. Bottom waters (blue triangle) are artificially given a depth of “−0.5 cm”. The sediment-water interface is indicated with a horizontal dashed line. Scales are consistent for each analyte, with the exception of (F), which has a different x-axis scale than (C) or (I).
In Experiment 2, porewater PO43− concentrations were broadly similar between non Flux, control, and Phoslock® cores, especially through the top 5 cm (Fig. 5, (D)). Although Core 6 non Flux was elevated relative to the others at depths (Fig. 5, (D), green diamond), all cores exhibited lower PO43− concentrations in the upper 5 cm relative to those cores sectioned for Experiment 1 (Fig. 5, (A) vs. (D)). For NH4+, we observed moderately higher concentrations in the top 3 cm for non Flux cores (p < 0.05), and Phoslock® cores also moderately elevated over Control cores (Fig. 5, (E)), although these trends did not continue with depths. Although studies with different experimental setups have associated elevated porewater NH4+ concentrations with reductions in nitrification rates due to Phoslock® treatment (Lin et al., 2017; Song et al., 2020), we are unable to say whether the elevated porewater NH4+ that we observed was due to Phoslock® itself, or due to the pulse of NH4+ to the overlaying water upon Phoslock® dispersal. The Control cores had much higher NO3− + NO2− concentrations at 0.5 cm (p < 0.001) than either the non Flux or Phoslock® cores (Fig. 5, (F), black), which is likely related to the lower NH4+ values due to increased nitrification under the oxic conditions.
In Experiment 3, PO43− concentrations in Control and Phoslock® cores were elevated relative to the non Flux cores in the first 0.5 cm fraction, however this difference did not continue down core (Fig. 5, (G), black and red vs. green), and it was also not significant (p > 0.1). However, because only data from the top 5 cm were available for Core 6 non Flux, we only have one full profile of a non Flux core for this experiment. NH4+ profiles were broadly similar across treatments (Fig. 5, (H), p > 0.1), however Core 5 Phoslock® had somewhat lower concentrations below 5 cm (Fig. 5, (H), red star). NO3− + NO2− concentrations were low regardless of treatment (Fig. 5(I)). For all three experiments, bottom water concentrations were generally lower than the first porewater 0.5 cm fraction for all analytes, with the exception of Experiment 2, in which the bottom water had the same PO43-− concentration as most porewater 0.5 cm fractions (Fig. 5, (D), blue triangle) and NO3− + NO2−was much higher than all porewater 0.5 cm fractions including the Control cores (Fig. 5, (F), blue triangle).
4. Discussion
Prior experimental determinations of the impact of Phoslock® on PO43− flux used cores packed with homogenized sediment, with the intention of reducing variability between cores (Egemose et al., 2010; Gibbs et al., 2011; Reitzel et al., 2013). However, homogenization also has the unintended effect of blurring the gradients near the sediment-water interface, and affects sediment physico-chemical properties, thereby artificially changing diffusive fluxes. Hence, homogenized cores are considered less representative of the natural lake sediments than intact cores. Our strategy of using some cores only for sectioning allows us to use intact cores and also address the variability and the impact of the experimental design on the results. We did so by conducting a two-factor ANOVA test (RStudio, aov Condition + Trial, type III) considering the impact of Trial number and condition (oxic control, oxic Phoslock®, anoxic control, anoxic Phoslock®) on sediment-water fluxes, which showed that mean PO43− fluxes were not significantly different between Trials (p>>0.05), despite significant differences based on condition (p<< 0.05). Given the visible similarity of our porewater profiles, and the lack of significant relationship between PO43− flux and trial number, we can conclude that our experimental design was reasonably effective at operating under steady-state conditions, allowing us to consider our pseudo-replicates as true replicates.
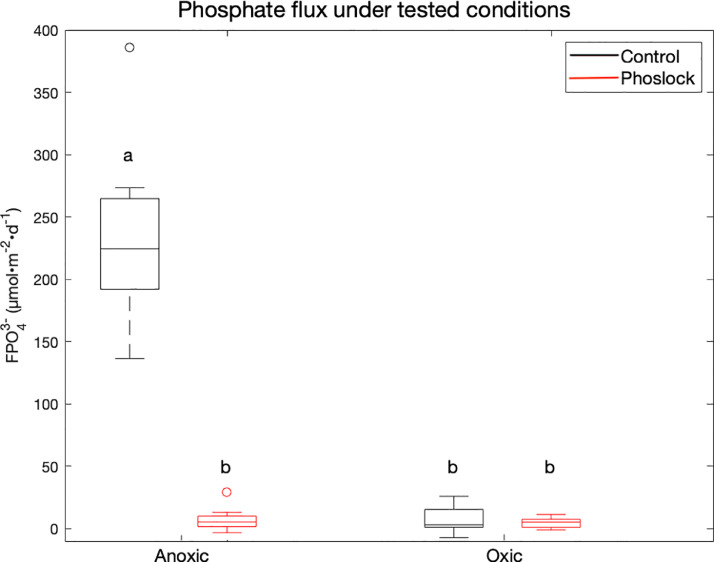
The primary goal of this study was to establish the relative efficacy of Phoslock® amendment and natural sedimentary iron under oxic and anoxic bottom water conditions, in clay-rich sediments. We found that, when the overlying water was oxygenated, PO43− flux was not statistically different (p >> 0.05) between the control (7.0 ± 11.4 µmol•m−2•d−1) and Phoslock® treatments (4.5 ± 4.3 µmol•m−2•d−1, Fig. 6, Oxic). These results are in contrast to a prior study in Lake Rotorua, a volcanic lake with very high internal P in New Zealand, where it was found that Phoslock® amendment could increase sediment uptake of DRP (dissolved reactive phosphate) under oxic conditions to approximately −12 mg•m−2•d−1 from approximately −2.5 mg•m−2•d−1 for the control (Gibbs et al., 2011). Our results are also in contrast with results from Lake Langesø, which indicated that Phoslock could improve P uptake from −44 µmol•m−2•d−1 (control) to 51 µmol•m−2•d−1. This highlights the importance of sediment and lake type when assessing the potential efficacy of Phoslock®. Jordan Lake's bottom waters often become suboxic in mid-summer, and under anoxic conditions, we found that Phoslock® treatment effectively decreased sediment PO43− fluxes (7.5 ± 9.5 µmol•m−2•d−1) relative to untreated sediment (236 ± 74 µmol•m−2•d−1, Fig. 6, Anoxic). This finding is similar to the results reported from Lake Rotorua (Gibbs et al., 2011), where a DRP flux of −8 mg•m−2•d−1 was observed with Phoslock® treatment, and 27 mg•m−2•d−1 flux for the control. The iron-rich Jordan Lake sediment creates a natural barrier to PO43− release, but only under oxic conditions, as PO43− bonds nearly irreversibly with Fe(III). This natural barrier to PO43− release was active during our study, as seen in the statistically indistinguishable PO43− fluxes between the Phoslock® and oxic control treatments (Fig. 6, p>>0.05). In other words, unamended Jordan Lake sediments are just as effective at reducing PO43− fluxes as sediments with Phoslock® treatment, provided that the bottom water is oxygenated. Jordan Lake experiences water quality issues throughout the year, even when the bottom water is oxygenated during the fall through spring (Cain, 2017; Wiltsie et al., 2018). Our results suggest that Phoslock® treatment will not mitigate these water quality issues when the water column is mixed, and may only be effective at reducing the intensity summer algal blooms.
Fig. 6.
Fluxes for PO43− in µmol•m−2•d−1, separated by treatment (Control, black and Phoslock®, red) and condition (Anoxic, left and Oxic, right). Flux calculations from all three experiments and trials are pooled.
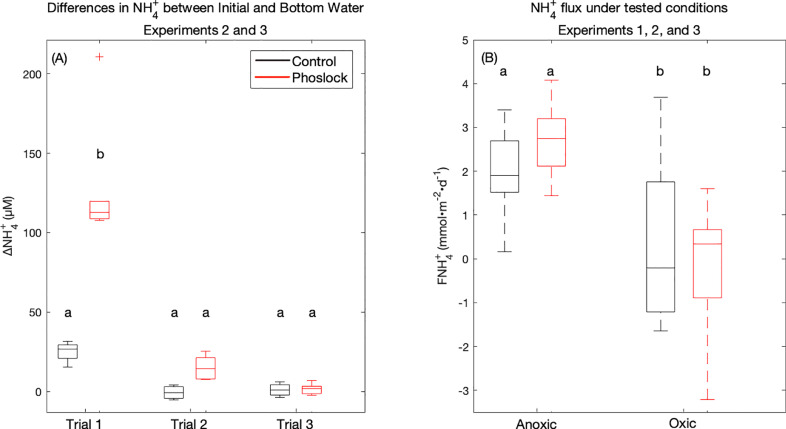
We also observed an unexpected and large release of NH4+ directly from Phoslock®. This NH4+ release was enough to increase the NH4+ concentration of the 150 mL overlying water by 129 ± 40 µM above bottom-water levels for the first trial (Fig. 7, (A), Trial 1 Red). This NH4+ release from Phoslock® could be a significant input of labile N to Jordan Lake. Assuming a 4.3 m water column (the average depth of Jordan Lake), the NH4+released from Phoslock® amounts to an increase in lake NH4+ concentration of 2.6 ± 0.8 μM. As the observed bottom water NH4+ concentrations ranged from 1.22 to 19.4 μM (Fig. 5, (B), (E), and (H), blue), this increase represents a roughly 10 to 275% increase in whole lake water column NH4+ concentrations. Using the NH4+ benthic flux for the anoxic controls (2.0 ± 1.0 mmol NH4+m−2 d−1; Fig. 7, (B) anoxic black), we can estimate that the sediment contribution of NH4+ increases water column concentrations by roughly 0.48 ± 0.24 µM d−1. In this case, the anoxic control NH4+ flux is used because the NH4+ flux is less affected by either nitrification or the pulse of NH4+ from Phoslock® treatment. Thus, Phoslock® could contribute the equivalent of roughly 2.5–14 days (median 5.5 days) of sediment NH4+ loading within minutes to hours of its application (although this estimate is calculated using our experimental results, from a temperature of 19–20 °C).
Fig. 7.
(A) The difference between the NH4+ concentration of the first time point (Initial) subtracted from the bottom water concentration at the start of each trial. Data is shown for Experiments 2 and 3, and separated by Trial (1, 2, or 3) and treatment (Control, black or Phoslock®, red), but is pooled by condition (Anoxic and Oxic). (B) The flux of NH4+ (mmol•m−2•d−1) calculated for each treatment (Control, black or Phoslock®, red) and condition (Anoxic, left and Oxic, right), but pooled by Trial.
A small increase in NH4+ was also observed in the control for the first trial (Fig. 7, (A) Trial 1, black), which was likely a result of the initial sample for this trial being collected 5–6 h after the addition of bottom water, to allow for Phoslock® settling, and could represent a 5–6-hour NH4+ flux. This factor was not present in subsequent trials (Fig. 5, (A) Trial 2 and Trial 3, black) which did not have such a delay, which indicates that our bottom water addition process was minimally disruptive to the sediment-water interface. As Phoslock® was only added prior to the first trial and overlying waters were replaced between trials, the impact of Phoslock® on the initial NH4+ concentration was greatly reduced for Trial 2 and eliminated for Trial 3 (Fig. 7, (A) Trial 2 and Trial 3, red).
Despite the increase in initial NH4+ concentrations, we saw sediment uptake of NH4+ under oxic conditions relative to anoxic conditions, and this difference persisted across Control and Phoslock® treatments (Fig. 7, (B)), suggesting rapid nitrification of NH4+ (Fig. 3, (B) and (C)). While the reduction in overlying water NH4+ throughout Experiment 2 suggests that this input of NH4+ could be rapidly cycled in an oxygenated Jordan Lake (Fig. 3, (B), red), there is reason to suspect that Phoslock® treatment could reduce the ability of the sediments to remove excess NH4+ through nitrification/denitrification processes relative to untreated lake sediment. Phoslock® has been shown to decrease the oxygen penetration depth in sediments, potentially into the Phoslock® layer itself for larger Phoslock® treatments (Vopel et al., 2008), which could decrease nitrification rates which occur in the thin oxygenated zone of sediments. Extended Phoslock® treatment has been linked to a reduction in nitrification rates, estimated either through increased porewater NH4+ (Song et al., 2020) or through reductions in abundance of archaeal ammonia-oxidizers (Lin et al., 2017). In our study, Phoslock® treated cores also showed elevated NH4+ and reduced NO3− + NO2− porewater concentrations relative to the Control cores (Fig. 5, (E) and (F), red vs. black), which provides some evidence for this effect. However, the results of Experiment 2 (Phoslock® under oxic conditions) offer mixed evidence of reduced nitrification with Phoslock®, as Phoslock® treated Cores 3 and 5 show reduced water column NO3− + NO2− relative to the Control Cores 1 and 2, while Core 4 Phoslock® shows elevated NO3− + NO2− (Fig. 3, (C)). As the batch incubation heightened the impact of the NH4+ input from Phoslock®, and as we did not measure N2 or quantify nitrification or denitrification rates, these results can only provide limited qualitative, rather than quantitative, support for Phoslock® reducing nitrification rates in lake sediments.
Our study is not the first to identify Phoslock® as a potential source of NH4+. For example, van Oosterhout et al. found that Phoslock® released NH4+ into nanopore water at a rate of 223 mg kg−1 Phoslock® (van Oosterhout and Lürling, 2013). Reitzel et al. reported that 4 grams of Phoslock® could release 10.3 mmol NH4+ m−2 when dispersed in Milli-Q water. However, they did not observe a corresponding increase in NH4+ when dispersed in their Lake Langesø water during their laboratory incubations, which they attributed to the lake's high alkalinity (Reitzel et al., 2013). Thus, the NH4+ release from Phoslock® may vary with lake water chemistry (especially alkalinity). However, our study shows that substantial NH4+ release is likely in Jordan Lake, and should be considered when deciding whether Phoslock® application is appropriate. This is especially true because as a reservoir, Jordan Lake is connected to the Cape Fear river and is upstream of the Cape Fear estuary, a potentially more N-limited system (Burkholder et al., 2006; Paerl, 2009). Simultaneously increasing N while effectively managing summertime P could have unintended consequences for Jordan Lake's N filtration ecosystem service (Finlay et al., 2013).
Our experiments did not fully address the reasons for NH4+ release from Phoslock®, but other studies have indicated that low alkalinity (Reitzel et al., 2013) could contribute to the release of NH4+, potentially due to an increase in the Phoslock® clay dispersion in soft water vs. hard waters with high Ca2+ concentration (Reitzel et al., 2017). If clay dispersion is the reason for this NH4+ release, then it is reasonable to expect that lanthanum may also be released with Jordan Lake water, although we did not measure it. Lanthanum is released under variable pH conditions (Ross et al., 2008), when humic or fulvic acid content is high (Dithmer et al., 2016; Reitzel et al., 2017; Reitzel et al., 2013; Wang et al., 2016), or when alkalinity is low (Reitzel et al., 2017; Spears et al., 2013). Our observed large release of NH4+ suggests that the Phoslock® matrix might not be stable in Jordan Lake waters and might be a source of heavy metal pollutants, including lanthanum.
5. Conclusion
This study tested the efficacy of Phoslock® in reducing internal phosphate nutrient loading from iron-rich sediments, in comparison with simple bottom water oxygenation. We found that Phoslock® did not provide an additional benefit compared to untreated sediment under conditions of bottom-water oxia in Jordan Lake (representative of fall through spring conditions), a piedmont reservoir in central North Carolina. However, during stratified summertime periods when Jordan Lake bottom waters experience anoxia, our results show that Phoslock® is more effective at lowering sediment phosphate fluxes, compared with the anoxic control without Phoslock®. This study did not test the long-term effectiveness of Phoslock®, which may decrease as sediments bury the Phoslock® amended layer. We also found that Phoslock® itself can be a source of ammonium when dispersed in Jordan Lake water. Our experimental treatment of 500 mg of Phoslock® per core increased NH4+concentration by 129 ± 40 µM in our 150 mL overlying waters, an equivalent to roughly 5.5 days of estimated sediment NH4+ loadings from untreated sediment. This NH4 release could exacerbate issues of N export to downstream areas associated with only focusing on P management strategies.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgements
The graphical abstract was made using images courtesy of the Integration and Application Network, University of Maryland Center for Environmental Science (ian.umces.edu/symbols/).
The authors would like to thank the North Carolina Department of Water Resources for their help in sampling. The authors are grateful to Bryce Van Dam, John S. Kominoski, and Jim Zeller for helpful edits and suggestions. The authors are grateful to West Bishop and SePRO corporation for providing Phoslock® and dosing suggestions, as well as to Thomas Blanchard and Sara Gay at WBAS.
Funding
This work was funded by the North Carolina Policy Collaboratory. Mary Zeller is currently supported within the BMBF project ‘DAM-MGF’.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.wroa.2021.100095. Data generated for this project is available at Figshare, doi:10.6084/m9.figshare.14159735.
Appendix. Supplementary materials
References
- Anderson H.S., Johengen T.H., Godwin C.M., Purcell H., Alsip P.J., Ruberg S.A., Mason L.A. Continuous In Situ Nutrient Analyzers Pinpoint the Onset and Rate of Internal P Loading under Anoxia in Lake Erie’s Central Basin. Environ. Sci. Tech. Water. 2021 doi: 10.1021/acsestwater.0c00138. https://doi.org/ [DOI] [Google Scholar]
- Berg P., Risgaard-Petersen N., Rysgaard S. Interpretation of measured concentration profiles in sediment pore water. Limnol. Oceanogr. 1998;43(7):1500–1510. doi: 10.4319/lo.1998.43.7.1500. https://doi.org/ [DOI] [Google Scholar]
- Berg P., Rysgaard S., Funch P., Sejr M.K. Effects of bioturbation on solutes and solids in marine sediments. Aquatic Microbial Ecology. 2001;26:81–94. doi: 10.3354/ame026081. [DOI] [Google Scholar]
- Berke P., Spurlock D., Hess G., Band L. Local comprehensive plan quality and regional ecosystem protection: the case of the Jordan Lake watershed, North Carolina, U.S.A. Land Use Policy. 2013;31:450–459. doi: 10.1016/j.landusepol.2012.08.009. https://doi.org/ [DOI] [Google Scholar]
- Bishop W.M., Richardson R.J. Influence of Phoslock® on legacy phosphorus, nutrient ratios, and algal assemblage composition in hypereutrophic water resources. Environ. Sci. Pollut. Res. 2018;25(5):4544–4557. doi: 10.1007/s11356-017-0832-2. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Bishop W.M., McNabb T., Cormican I., Willis B.E., Hyde S. Operational evaluation of Phoslock phosphorus locking technology in Laguna Niguel Lake, California. Water Air Soil Pollut. 2014;(7):225. doi: 10.1007/s11270-014-2018-6. https://doi.org/ [DOI] [Google Scholar]
- Burkholder J.A.M., Dickey D.A., Kinder C.A., Reed R.E., Mallin M.A., McIver M.R. Comprehensive trend analysis of nutrients and related variables in a large eutrophic estuary: a decadal study of anthropogenic and climatic influences. Limnol. Oceanogr. 2006;51(1 II):463–487. doi: 10.4319/lo.2006.51.1_part_2.0463. https://doi.org/ [DOI] [Google Scholar]
- Cain J.L. North Carolina State University; 2017. Water Quality and Stratification in Jordan Lake: Assessment of Spatial, Temporal and Inter-Annual Variability. Retrieved from https://repository.lib.ncsu.edu/bitstream/handle/1840.20/34550/etd.pdf?sequence=1. [Google Scholar]
- Copetti D., Finsterle K., Marziali L., Stefani F., Tartari G., Douglas G. Eutrophication management in surface waters using lanthanum modified bentonite: a review. Water Res. 2015;97:162–174. doi: 10.1016/j.watres.2015.11.056. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Dithmer L., Nielsen U.G., Lundberg D., Reitzel K. Influence of dissolved organic carbon on the efficiency of P sequestration by a lanthanum modified clay. Water Res. 2016;97:39–46. doi: 10.1016/j.watres.2015.07.003. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Dithmer L., Nielsen U.G., Lürling M., Spears B.M., Yasseri S., Lundberg D. Responses in sediment phosphorus and lanthanum concentrations and composition across 10 lakes following applications of lanthanum modified bentonite. Water Res. 2016;97:101–110. doi: 10.1016/j.watres.2016.02.011. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Dodds W.K., Perkin J.S., Gerken J.E. Human impact on freshwater ecosystem services: a global perspective. Environ. Sci. Technol. 2013;47(16):9061–9068. doi: 10.1021/es4021052. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Egemose S., Reitzel K., Andersen F., Flindt M.R. Chemical lake restoration products: sediment stability and phosphorus dynamics. Environ. Sci. Technol. 2010;44(3):985–991. doi: 10.1021/es903260y. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Finlay J.C., Small G.E., Sterner R.W. Human Influences on Nitrogen Removal in Lakes. Science. 2013;342:247–250. doi: 10.1126/science.1242575. October https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Gächter R., Müller B. Why the phosphorus retention of lakes does not necessarily depend on the oxygen supply to their sediment surface. Limnol. Oceanogr. 2003;48(2):929–933. doi: 10.4319/lo.2003.48.2.0929. https://doi.org/ [DOI] [Google Scholar]
- Gibbs M.M., Hickey C.W., Özkundakci D. Sustainability assessment and comparison of efficacy of four P-inactivation agents for managing internal phosphorus loads in lakes: sediment incubations. Hydrobiologia. 2011;658(1):253–275. doi: 10.1007/s10750-010-0477-3. https://doi.org/ [DOI] [Google Scholar]
- Hanway J.J., Scott A.D., Stanford G. Replaceability of Ammonium Fixed in Clay Minerals as Influenced by Ammonium or Potassium in the Extracting Solution. Soil Sci. Soc. Am. J. 1957;21(1):29–34. doi: 10.2136/sssaj1957.03615995002100010008x. https://doi.org/https://doi.org/ [DOI] [Google Scholar]
- Lin J., Zhong Y., Fan H., Song C., Yu C., Gao Y. Chemical treatment of contaminated sediment for phosphorus control and subsequent effects on ammonia-oxidizing and ammonia-denitrifying microorganisms and on submerged macrophyte revegetation. Environ. Sci. Pollut. Res. 2017;24(1):1007–1018. doi: 10.1007/s11356-016-7828-1. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Lürling M., Faassen E.J. Controlling toxic cyanobacteria: effects of dredging and phosphorus-binding clay on cyanobacteria and microcystins. Water Res. 2012;46(5):1447–1459. doi: 10.1016/j.watres.2011.11.008. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Lürling M., Waajen G., Engels B., van Oosterhout F. Effects of dredging and lanthanum-modified clay on water quality variables in an enclosure study in a hypertrophic pond. Water. 2017;9(6):380. doi: 10.3390/w9060380. https://doi.org/ [DOI] [Google Scholar]
- Le Moal M., Gascuel-Odoux C., Ménesguen A., Souchon Y., Étrillard C., Levain A. Eutrophication: a new wine in an old bottle? Sci. Total Environ. 2019;651:1–11. doi: 10.1016/j.scitotenv.2018.09.139. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Moss B., Jeppesen E., Søndergaard M., Lauridsen T.L., Liu Z. Nitrogen, macrophytes, shallow lakes and nutrient limitation: resolution of a current controversy? Hydrobiologia. 2013;710(1):3–21. doi: 10.1007/s10750-012-1033-0. https://doi.org/ [DOI] [Google Scholar]
- Murniati E., Gross D., Herlina H., Hancke K., Lorke A. Effects of bioirrigation on the spatial and temporal dynamics of oxygen above the sediment-water interface. Freshwater Science. 2017;36(4):784–795. doi: 10.1086/694854. https://doi.org/ [DOI] [Google Scholar]
- O’Dell J.W. U.S. Environmental Protection Agency; 1993. Method 350.1 Determination of Ammonia Nitrogen by Semi-Automated Colorimetry. [Google Scholar]
- van Oosterhout F., Lürling M. The effect of phosphorus binding clay (Phoslock®) in mitigating cyanobacterial nuisance: a laboratory study on the effects on water quality variables and plankton. Hydrobiologia. 2013;710(1):265–277. doi: 10.1007/s10750-012-1206-x. https://doi.org/ [DOI] [Google Scholar]
- Van Oosterhout F., Goitom E., Roessink I., Lürling M. Lanthanum from a modified clay used in eutrophication control is bioavailable to the marbled crayfish (Procambarus fallax f. virginalis) PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0102410. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihel D.M., Schindler D.W., Ballard N.C., Graham M.D., O’Connell D.W., Wilson L.R., Vinebrooke R.D. The “nutrient pump:” Iron-poor sediments fuel low nitrogen-to-phosphorus ratios and cyanobacterial blooms in polymictic lakes. Limnol. Oceanogr. 2015;60(3):856–871. doi: 10.1002/lno.10076. https://doi.org/ [DOI] [Google Scholar]
- Orihel D.M., Schindler D.W., Wilson L.R., Vinebrooke R.D., Ballard N.C. Experimental iron amendment suppresses toxic cyanobacteria in a hypereutrophic lake. Ecol. Appl. 2016;26(5):1517–1534. doi: 10.1890/15-1928. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Paerl H.W. Controlling eutrophication along the freshwater-Marine continuum: dual nutrient (N and P) reductions are essential. Estuaries Coasts. 2009;32(4):593–601. doi: 10.1007/s12237-009-9158-8. https://doi.org/ [DOI] [Google Scholar]
- Paerl H.W., Hall N.S., Calandrino E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011;409(10):1739–1745. doi: 10.1016/j.scitotenv.2011.02.001. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Reitzel K., Lotter S., Dubke M., Egemose S., Jensen H.S., Andersen F. Effects of Phoslock® treatment and chironomids on the exchange of nutrients between sediment and water. Hydrobiologia. 2013;703(1):189–202. doi: 10.1007/s10750-012-1358-8. https://doi.org/ [DOI] [Google Scholar]
- Reitzel K., Andersen F.T., Egemose S., Jensen H.S. Phosphate adsorption by lanthanum modified bentonite clay in fresh and brackish water. Water Res. 2013;47(8):2787–2796. doi: 10.1016/j.watres.2013.02.051. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Reitzel K., Balslev K.A., Jensen H.S. The influence of lake water alkalinity and humic substances on particle dispersion and lanthanum desorption from a lanthanum modified bentonite. Water Res. 2017;125:191–200. doi: 10.1016/j.watres.2017.08.044. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Renz J.R., Powilleit M., Gogina M., Zettler M.L., Morys C., Forster S. Community bioirrigation potential (BIPc), an index to quantify the potential for solute exchange at the sediment-water interface. Mar. Environ. Res. 2018;141:214–224. doi: 10.1016/j.marenvres.2018.09.013. Julyhttps://doi.org/ [DOI] [PubMed] [Google Scholar]
- Ross G., Haghseresht F., Cloete T.E. The effect of pH and anoxia on the performance of Phoslock®, a phosphorus binding clay. Harmful Algae. 2008;7(4):545–550. doi: 10.1016/j.hal.2007.12.007. https://doi.org/ [DOI] [Google Scholar]
- Rothe M., Frederichs T., Eder M., Kleeberg A., Hupfer M. Evidence for vivianite formation and its contribution to long-term phosphorus retention in a recent lake sediment: a novel analytical approach. Biogeosciences. 2014;11(18):5169–5180. doi: 10.5194/bg-11-5169-2014. https://doi.org/ [DOI] [Google Scholar]
- Schindler David W., Hecky R.E., Findlay D.L., Stainton M.P., Parker B.R., Paterson M.J., Beaty K.G., Lyng M., Kasian S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. 2008;105(32):11254–11258. doi: 10.1073/pnas.0805108105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler D.W. The dilemma of controlling cultural eutrophication of lakes. Proc. R. Soc. B. 2012;279(1746):4322–4333. doi: 10.1098/rspb.2012.1032. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler D.W., Carpenter S.R., Chapra S.C., Hecky R.E., Orihel D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016;50(17):8923–8929. doi: 10.1021/acs.est.6b02204. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Smith V.H., Joye S.B., Howarth R.W. Eutrophication of freshwater and marine ecosystems. Limnol. Oceanogr. 2006;51(1, part2):351–355. doi: 10.4319/lo.2006.51.1_part_2.0351. https://doi.org/ [DOI] [Google Scholar]
- Søndergaard M., Bjerring R., Jeppesen E. Persistent internal phosphorus loading during summer in shallow eutrophic lakes. Hydrobiologia. 2013;710(1):95–107. doi: 10.1007/s10750-012-1091-3. https://doi.org/ [DOI] [Google Scholar]
- Song X., Li D., Zhao Z., Zhou J., Xu C., Geng X., Huang Y. The effect of microenvironment in the sediment on phosphorus immobilization under capping with ACPM and Phoslock®. Environ Sci Pollut Res. 2020;27(13):15440–15453. doi: 10.1007/s11356-020-08105-8. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Spears B.M., Lürling M., Yasseri S., Castro-Castellon A.T., Gibbs M., Meis S. Lake responses following lanthanum-modified bentonite clay (Phoslock®) application: an analysis of water column lanthanum data from 16 case study lakes. Water Res. 2013;47(15):5930–5942. doi: 10.1016/j.watres.2013.07.016. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Spears B.M., Mackay E.B., Yasseri S., Gunn I.D.M., Waters K.E., Andrews C. A meta-analysis of water quality and aquatic macrophyte responses in 18 lakes treated with lanthanum modified bentonite (Phoslock®) Water Res. 2016;97:111–121. doi: 10.1016/j.watres.2015.08.020. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Vopel K., Gibbs M., Hickey C.W., Quinn J. Modification of sediment-water solute exchange by sediment-capping materials: effects on O2 and pH. Mar. Freshwater Res. 2008;59(12):1101–1110. doi: 10.1071/MF08130. https://doi.org/ [DOI] [Google Scholar]
- Vymazal J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007;380(1–3):48–65. doi: 10.1016/j.scitotenv.2006.09.014. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Waajen G., van Oosterhout F., Lürling M. Bio-accumulation of lanthanum from lanthanum modified bentonite treatments in lake restoration. Environ. Pollut. 2017;230:911–918. doi: 10.1016/j.envpol.2017.07.046. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Waajen Guido, van Oosterhout F., Douglas G., Lürling M. Geo-engineering experiments in two urban ponds to control eutrophication. Water Res. 2016;97:69–82. doi: 10.1016/j.watres.2015.11.070. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Wang C., Bai L., Jiang H.L., Xu H. Algal bloom sedimentation induces variable control of lake eutrophication by phosphorus inactivating agents. Sci. Total Environ. 2016;557–558:479–488. doi: 10.1016/j.scitotenv.2016.03.082. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Wang C., Jiang H.-.L., Xu H., Yin H. Variation of physicochemical properties of drinking water treatment residuals and Phoslock((R)) induced by fulvic acid adsorption: implication for lake restoration. Environ Sci Pollut Res Int. 2016;23(1):351–365. doi: 10.1007/s11356-015-5209-9. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Wiltsie D., Schnetzer A., Green J., Borgh M.Vander, Fensin E. Algal blooms and cyanotoxins in Jordan Lake, North Carolina. Toxins (Basel) 2018;10(2) doi: 10.3390/toxins10020092. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtsbaugh W.A., Paerl H.W., Dodds W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdisciplinary Reviews: Water. 2019;6(5):1–27. doi: 10.1002/wat2.1373. https://doi.org/ [DOI] [Google Scholar]
- Zamparas M., Zacharias I. Restoration of eutrophic freshwater by managing internal nutrient loads. A review. Sci. Total Environ. 2014;496:551–562. doi: 10.1016/j.scitotenv.2014.07.076. https://doi.org/ [DOI] [PubMed] [Google Scholar]
- Zhang J.-.Z., Ortner P.B., Fischer C.J. U.S. Environmental Protection Agency; 1997. Method 353.4 Determination of Nitrate and Nitrite in Estuarine and Coastal Waters By Gas Segmented Continuous Flow Colorimetric Analysis. [Google Scholar]
- Zimmermann C.F., Keefe C.W. Method 365.5 Determination of Orthophosphate in Estuarine and Coastal Waters by Automated Colorimetric Analysis. U.S. Environmental Protection Agency. 1997 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.