Abstract
BACKGROUND AND PURPOSE: Functional MR (fMR) imaging data coregistered to a neurosurgical navigation system have been proposed as guides for the resection of brain tumor in or adjacent to eloquent cortices. The purpose of this study was to compare data obtained from the side of the brain affected by tumor with the contralateral side and to determine if there are physiological limitations of fMR imaging in accurately determining the location of the primary motor cortex.
METHODS: Ten patients with tumors in or directly adjacent to the motor cortex were studied with fMR imaging (finger-tapping paradigm). fMR imaging data were analyzed using multiple R values. These data were coregistered to a real-time intraoperative neurosurgical navigation system.
RESULTS: Significant variability of motor cortex activation patterns was noted among individual patients. The activation volumes on the side of the tumor were significantly smaller compared with the contralateral side for all tumors not previously resected (0.66±0.47). This was most pronounced in glioblastomas (0.27±0.21). We propose that these differences were caused by a loss of autoregulation in the tumor vasculature of glioblastomas and venous effects.
CONCLUSION: Notwithstanding the differences noted, the motor cortex was identified successfully in all patients. This was confirmed by intraoperative physiological identification of the motor cortex and a lack of postoperative neurologic deficit.
The goal of brain tumor surgery is to maximize tumor resection while preserving important brain function (1–3). It has been shown that the length and quality of survival among patients with brain tumors is improved with maximized tumor resection in both malignant gliomas and menigiomas (1, 2, 4–6). In order to preserve vital neurologic function controlled by the brain tissue directly adjacent to or involved by the tumor, it is important for the neurosurgeon to be able to identify the anatomic location of this eloquent cortex intraoperatively. Morphologic identification of the eloquent cortices to be avoided during surgery is complicated by distortion of the anatomy of mass lesions (3, 7–10) and by the fact that the functional cortex does not always correspond to its expected anatomic location (3, 10–15). Traditionally, the eloquent cortices, such as the motor cortex, have been identified intraoperatively by physiological methods such as direct cortical stimulation or somatosensory evoked potentials (2).
A number of investigators have reported that presurgical blood-oxygen-level–dependent (BOLD) functional MR (fMR) imaging can be used to define functional cortices of the brain in preoperative planning for glioma and meningioma surgery (1, 2, 16, 17). Methods based on preoperative identification of the eloquent cortices by functional imaging would be advantageous, because they avoid certain difficulties encountered by intraoperative methods. First, identification of the eloquent cortices would occur preoperatively, which would decrease the length of surgery and the time the patient would be under anesthesia. Second, intraoperative identification of some eloquent cortices, such as the language cortex, requires the patient to be awake during the operation (2). Third, intraoperative physiological methods are occasionally unsuccessful.
Before preoperative methods based on fMR imaging can replace the current standard, however, the accuracy of the former must be established. Analysis of fMR imaging data is based on statistical methods. It is axiomatic that application of different statistical parameters to define the volume of the eloquent cortex in question would lead to different results. For example, in evaluating the motor cortex, if one uses a lower correlation coefficient (R value) or a higher significance (P value), the volume of activation, which defines the motor cortex, would be larger. This raises a fundamental question of critical importance to the surgeon, especially when the tumor to be resected coincides with one volume of activation but not with the other: which R and P value is optimal to define the volume of activation?.
A recent report (18) has raised a number of questions that may limit the accuracy of BOLD fMR imaging in defining eloquent cortices adjacent to brain tumors. It is known that there is a loss of autoregulation of the vasculature of malignant gliomas. Angiographic and MR studies have shown that the blood vessels of a glioma respond much less vigorously to a number of physiological stimuli. BOLD fMR imaging is based on there being a difference in the volume of blood flow and the concentration of deoxyhemoglobin in the area of activation with increased neural activity. If this loss of autoregulation of the glioma blood vessels extends to the inability of blood vessels to respond normally to increased neural activity, this would limit the ability of BOLD fMR imaging to define accurately the area of increased neural activity in an eloquent cortex involved by a glioma. Herein, we shall compare the volumes of activation between the side of the brain with tumor and the contralateral side by using different statistical parameters to determine if there is a difference between the activation patterns.
Methods
Ten patients were studied, of whom five had menigiomas, three glioblastoma multiforme, one oligodendroglioma, and one metastasis (Table). In all of the patients, the tumor was either in or was directly adjacent to the primary motor cortex. All patients underwent a physical examination, with specific attention paid to the neurologic function elicited from the area of the brain that was being considered for surgery. All patients obtained a routine anatomic MR examination within 3 weeks prior to the fMR scan and surgery. Imaging included an axial T1-weighted (500/14/1 [TR/TE/excitations]), balanced (2500/30/0.5), and T2-weighted (2500/85/0.5) scans. After the IV administration of 0.1 mmol/kg of Gd-DTPA, axial (500/14), T1-weighted images were obtained. All of the scans were performed on a 1.5-T unit. The matrix size was 256 × 256, the field of view was 230 mm, and the slice thickness was 5 mm with a 1 mm interslice gap.
Quantitative data from functional and anatomic MR images in patients with tumors in or adjacent to the motor cortex
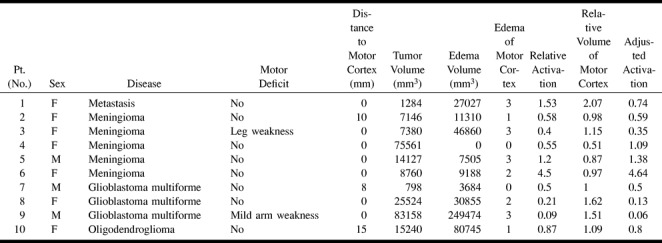
Quantitative data were obtained for the volume of peritumoral edema by drawing regions of interest around the areas of T2 signal abnormality that were outside the tumor mass on each slice. To obtain the volume of the tumor itself, a region of interest was drawn around the area of enhancement for all tumors that exhibited significant enhancement (all of the tumors except the oligodendroglioma). The volume of the oligodendroglioma was obtained by measuring the tumor mass, as seen on the T2-weighted image. The volume of the motor cortex itself was also determined for each patient by drawing a region of interest of the entire precentral gyrus, not only the volume of activation. For each volumetric measurement (peritumoral edema, volume of the tumor, and volume of the motor cortex), the area of each region of interest was multiplied by the slice thickness plus the interslice gap, to give a volume for each slice. The volumes of every slice were then added to give the total volume.
A qualitative assessment was performed as to the degree of involvement of the motor cortex (as defined by the fMR study and later confirmed by intraoperative methods) by peritumoral edema. A value of 3 indicated marked involvement, a 2 indicated moderate involvement, a 1 indicated questionable involvement, and a 0 indicated no involvement.
The closest distance from the tumor to the motor cortex was determined. The motor cortex was defined as the gyrus indicated to be the primary motor cortex by the fMR study. For the purpose of this measurement, the tumor was defined as the area of enhancement for all tumor exhibiting significant enhancement (all of the tumors except the oligodendroglioma). For the oligodendroglioma, the tumor was defined as the mass as seen on the T2-weighted image. It is acknowledged that in glial tumors, malignant cells are identified in biopsy material far past the borders of enhancement.
fMR imaging was performed to define the location of motor cortex in relation to the lesion, and to register it to a surgical navigation system for real-time intraoperative localization, as described by Maldjian et al and Schulder et al (1, 2). The fMR scan was obtained 1 day prior to surgery. The patient performed a finger-tapping paradigm to activate the motor cortex. This consisted of alternating periods of 30 seconds of rest and 30 seconds of bilateral self-paced finger tapping for a total of 3 periods of rest and 2 periods of activation. The patient practiced the paradigm before the scan. The patient was observed to make sure that s/he performed the paradigm accurately during acquisition of the fMR imaging data. At the time of the scan, none of the patients exhibited any hand weakness. No difference was observed between the right and left hands during the patients' performance of the motor paradigms.
The functional data were acquired using the BOLD technique (gradient-echo echo-planar imaging, 2000/60 [TR/TE], 14 slices, 64 × 64 matrix, 5-mm slice thickness with no gap). A contrast-enhanced (Gd-DTPA 0.1 mmol/kg) T1-weghted scan (500/14 [TR/TE], 256 × 256 matrix, 230-mm field of view, and 3-mm slice thickness with no gap) was obtained immediately afterward.
The raw fMR data were analyzed off line using a SPARC20 workstation and software written in IDL, as previously described (1, 2). fMR maps were generated using a cross-correlation technique (19) for multiple R values ranging from 0.90 to 0.40, at an interval of every 0.05. For every R value, a region of interest was drawn around the area of activation in the primary motor cortex, as defined by the fMR data. In drawing the regions of interest of the primary motor cortex, care was taken not to include the activation that was frequently noted in the sensory cortex and in the supplementary motor area. Areas of activation directly adjacent to but outside the motor gyrus itself were also excluded, because they may have represented contamination from cortical veins.
For each patient, an optimal R value was determined. This determination was based on the subjective evaluation by a fellowship-trained neuroradiologist regarding which R value best demonstrated the motor cortex while minimizing activation in the area in the brain thought to represent noise. The ratio of the volume of activation between the side with the tumor and the contralateral side was determined for the optimal R value as well as for R values 0.05 above and below the optimal R value. A value termed the relative activation was determined by averaging the ratios of the optimal R value with the R values 0.05 above and below the optimal R value and is illustrated in the following equation:
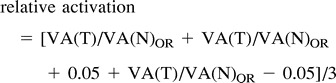 |
where: VA(T) = volume of activation in the motor cortex on the side with the tumor, VA(N) = volume of activation in the motor cortex on the side without the tumor, OR = at the optimal R value for each specific patient, and OR+0.05 = at the optimal R value + 0.05 for each specific patient, and OR-0.05 = at the optimal R value− 0.05 for each specific patient
The relative volume of the motor cortex was determined for each patient by normalizing the volume of the side with the tumor to the contralateral side.
where: Vmc(T) = volume of the motor cortex on the side with the tumor and Vmc(N) = volume of the motor cortex on the side without the tumor
For each patient, an adjusted activation, defined as the relative activation normalized to the ratio of the volumes of the motor cortices, was determined.
 |
The fMR data using the optimal R value was coregistered to the high-resolution contrast-enhanced scan. These fused images were transferred to a neurosurgical navigation system over an ethernet connection. During the operation, the neurosurgeon was able to define any point on the brain by the use of a probe. The defined point was displayed simultaneously in real time in three orthogonal plains and in a 3D rendering. Because the fMR data identifying the primary motor cortex were coregistered to the anatomic images, the neurosurgeon was able to identify not only the anatomy but also the functional areas of the brain by using the display in the operating room (1, 2). The neurosurgeon was able to visualize the relationship of the point defined by the probe to the tumor and to the adjacent primary motor cortex. The motor cortex was also identified by physiological methods including direct cortical stimulation and somatosensory evoked potentials during the operation. Owing to ethical considerations and institutional review board restrictions, the volume of resection was determined by the intraoperative physiological methods. Histologic confirmation was obtained for all of tumors. After surgery, the patients were assessed both during the immediate postoperative period and by multiple follow-up examinations to determine whether they had undergone neurologic deterioration, which could be attributed to resection of functioning eloquent cortex.
Results
The gyrus, which represented the primary motor cortex, was identified in every case by the intraoperative physiological methods. In every case, this correlated to the gyrus, which was predicted by the preoperative fMR scans. Postoperatively, none of the patients suffered any new neurologic deficits. All cases were confirmed histologically.
General Considerations in Defining the Motor Cortex
Notwithstanding the ability of fMR to predict successfully the primary motor gyrus, there was a wide variability in a number of factors used to define the motor cortex (Table). This included the optimal R value, the volume of activation at the optimal R value, the first R value where activation was identified, and the pattern of activation at different R values. The optimal R value had an average ±SD of 0.58±0.09. The range was 0.75 to 0.45. The average ±SD of the R value at which activation first occurred was 0.71 ±0.09. The range was 0.85 to 0.55. The mean ±SD of the volume of activation at the optimal R value was 1,260±412 mm3. The range was 816 to 1905 mm3. A large variation was also noted in the in the volume of activation at P<.05. The mean ±SD is 3009±1327 mm3.
Differences between the Hemisphere with the Tumor and the Contralateral Hemisphere
Significant differences were noted in the activation patterns between the hemisphere with the tumor and the contralateral hemisphere. Counting all patients, the mean ±SD of the relative activation was 1.04±1.29. If one excludes the only case that was operated on previously (case 6), the mean ±SD of the relative activation was 0.66 ± 0.47. The mean relative activation ±SD was the smallest for the glioblastoma multiforme group (0.27± 0.21) (Fig 1 and 2). Therefore, of all the pathologic entities, the difference between the volume of activation on the side with the tumor and the contralateral side was greatest for glioblastoma multiformes. For the meningiomas, the relative activation ±SD was 1.45±1.73. If one excludes the case operated on previously (case 6), then the relative activation ±SD was 0.68±0.35.
fig 1.
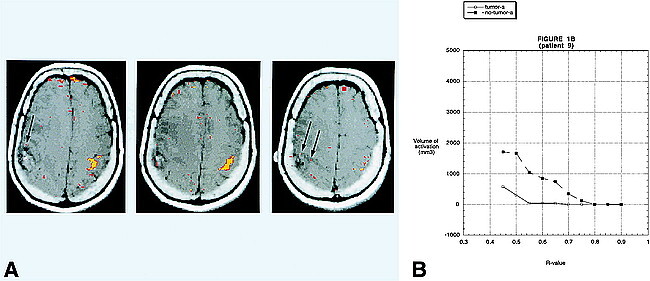
A, BOLD fMR activation from a finger-tapping paradigm coregistered to an axial T1-weighted image (500/14 [TR/TE]) in patient 9. Pathologic analysis revealed a right parietal lobe glioblastoma multiforme. The areas in yellow correspond to an R value of 0.65. The areas in red correspond to an R value of 0.50. There is robust activation in the left motor cortex. The activation in the right motor cortex is barely perceptible (arrows).
B, A graph of the volume of activation for different R values for patient 9. For all R values that reveal activation, the volume of activation is greater on the side opposite the tumor.
Significant differences in the volume of the motor cortex itself were noted in four patients. In patient 4, a large meningioma caused marked physical compression of the adjacent motor cortex, leading to decreased volume of the motor cortex on the side with the tumor relative to the contralateral side by a factor of 0.51 (Table). In three cases, there was a prominent degree of edema, which led to a difference in the volume of the motor cortex. Two of these cases were glioblastomas (patient 8 and 9), and one was a metastasis (patient 1). The ratio of the volumes of the motor cortices was 1.62 (patient 8) and 1.51 (patient 9) for the glioblastomas and 2.07 for the metastasis (Table). For the other six patients, the volumes of the two motor cortices were within 15% of each other.
The measurement of adjusted activation (the relative activation adjusted for the differences in the volume of the motor cortex itself) is displayed in the Table. Counting all patients, the mean ±SD of the relative activation was 1.03±1.33. If one excludes the only case that was operated on previously (case 6), the mean ±SD of the relative activation was 0.63±0.43. The adjusted activation ±SD was also the smallest for the glioblastoma multiforme group (0.23±0.24). For the meningiomas, the adjusted activation ±SD was 1.61±1.74. If one excludes the case operated on previously, the relative activation ±SD was 0.85±0.47.
Discussion
A real-time intraoperative system, which allows the neurosurgeon to visualize not only the morphologic features of the brain directly, including the pathologic features, but also accurately delineate functional cortices adjacent to the tumor to be resected, would be of obvious benefit. Many such systems, some based on BOLD fMR imaging (1, 2, 20, 21) and others on different imaging techniques (22, 23), have been proposed. In order for any such system to become accepted for general use, its accuracy must be established. In addition, possible limitations of a proposed system and the conditions in which these limitations occur must also be defined.
In this study, we present the results of 10 patients with brain tumors in or directly adjacent to the primary motor cortex. In these patients, the motor cortex was defined by BOLD fMR imaging. fMR data were confirmed intraoperatively by physiological methods and by postoperative assessment of neurologic status. Using the same statistical parameters, the results indicate that there is a considerable variation in the volumes of activation in the primary motor cortices of different patients. In addition, there appears to be a significant difference in the volumes of activation between the side of the brain with the tumor and the contralateral side.
The discussion will focus first on possible reasons for the differences observed in the volume of activation between the side with the tumor and the contralateral side. Specifically, the possible causes for such a difference and whether this represents a fundamental limitation of this method will be explored. Secondly, general principles on the use of statistical analysis to optimize localization of the primary motor cortex will be investigated.
Differences in the Volume of Activation between the Hemisphere with the Tumor and the Contralateral Hemisphere
The results indicate that there are significant differences between the volumes of activation on the side with the tumor and the contralateral side. This difference was most pronounced in the cases of glioblastoma multiforme (Fig 1 and 2). The adjusted activation ±SD was 0.23±0.24. All of the glioblastoma multiformes had relative and adjusted activations less than 0.5. The glioblastoma with the greatest relative and adjusted activation was a very small tumor with a volume of less than 1 cm3 and a volume of surrounding edema of 3.7 cm3. The other glioblastomas had relative and adjusted activations that were all less than or equal to 0.21. This means that the ratio of the volume of activation on the side with the tumor to the contralateral side was almost 5:1. Meningiomas exhibited a great variability in both the relative activation and in the adjusted activation. The mean ±SD for meningiomas was 1.45±1.73 (relative activation) and 1.61±1.74 (adjusted activation). A number of reasons will be considered as the possible causes of the described difference. These include the loss of autoregulation, contribution of the venous component, effect of edema, and the size of the tumor.
In case number 4 (Fig 3), a meningioma, there was prominent activation of the motor cortex on the side contralateral to the tumor at R values of 0.65, 0.70, and 0.75 that allowed for accurate delineation of the primary motor cortex. At these R values, there was very little activation on the side with the tumor. At R values of 0.50, 0.55, and 0.60, however, the activation in the primary motor cortex approached the activation on the contralateral side. This occurred before the onset of noise in areas of the brain not related to the neural control of motor function. This example emphasizes the need to evaluate each patient individually at multiple R values.
fig 3.
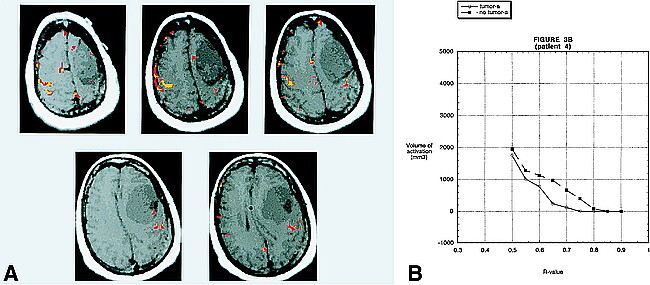
A, BOLD fMR activation from a finger-tapping paradigm coregistered to an axial T1-weighted image (500/14 [TR/TE]) in patient 4. Pathologic analysis revealed a left frontal lobe meningioma. There is prominent activation of the motor cortex on the side contralateral to the tumor, at R values of 0.70 (yellow) that allows for accurate delineation of the primary motor cortex. At these R values, there is very little activation on the side with the tumor. At R values of 0.55 (red), however, the volume of activation in the primary motor cortex of the side with the tumor approaches the activation on the contralateral side. This occurs before the onset of “noise” in areas of the brain not related to the neural control of motor function. This example emphasizes the need to evaluate each patient individually at multiple R values. Areas of activation located outside the brain over the right frontal convexity probably represent cortical venous drainage.
B, A graph of the volume of activation for different R values for patient 4. There is a large difference in the volume of activation between the two sides for R values between 0.65 and 0.75. At 0.50, however, the volume of activation on the two sides is almost the same.
Loss of Autoregulation
The difference in the volume of activation between the two sides was most pronounced in glioblastomas. It is possible that the phenomenon described in this report may be accounted for by the following mechanism. It is known from both angiographic as well as MR studies that tumor vasculature in malignant gliomas loses the ability of autoregulation. In angiographic studies, the vasculature of gliomas revealed an abnormal response to various physiological and pharmacological challenges including hypocapnia induced by hyperventilation (24, 25), hypercapnia (25), induced hypertension (26), and papavarine injection (26).
BOLD fMR imaging is based on the premise that increased neural activity is inherently linked both temporally and spatially to an increase in blood flow and resultant changes in deoxyhemoglobin concentration (27). If, however, the brain's ability to autoregulate the flow of blood is lost in brain tissue, which is still functioning, then this area may not respond to increased neural activity by a corresponding increase in blood flow. Consequently, the area in question may not show a statistically significant change on the BOLD fMR images. What may occur in this situation is that the tumor vasculature in the motor cortex has diminished or absent capability for autoregulation. This may preclude an increase in blood flow in the expected area of activation that normally occurs secondary to motor activity. A lack of increased blood flow to the expected area of activation would significantly limit the ability of BOLD fMR imaging to detect activation.
It is not known whether the loss of autoregulation of the vasculature of glioblastomas to the aforementioned challenges connotes that there is also a loss of autoregulation with increased neural activity related to finger tapping. Many factors, however, seem to support this contention. First, it appears that the effect described herein is most prominent in intraaxial tumors. One would not expect the loss of autoregulation described for gliomas to affect extraaxial tumors. It would seem unlikely that a tumor that, for the most part, exists outside the brain would affect the autoregulation properties of vessels, which are physically removed from the tumor itself. Secondly, the loss of autoregulation described in the literature for the glial tumors was much more prominent in glioblastoma multiforme than in more benign glial tumors (24, 26). The data from this investigation demonstrate that the difference in the volume of activation between the two sides was much more pronounced in glioblastoma multiforme (patients 7, 8, and 9) than in the patient with the more benign intraaxial tumor (patient 10).
Contribution of a Venous Component
A significant percentage, if not the majority, of the signal change from BOLD fMR images is due to a change in the oxygenation state of the hemoglobin in the venules and larger veins, as opposed to the capillaries (28, 29). Because the venules, and especially the larger veins, are removed from the exact location of the neural activation, it follows that BOLD fMR signal may not be an exact spatial representation (albeit by a rather small distance) of the actual brain activation. This fact has been a cause of uncertainty as to the exact correlation of the BOLD fMR signal to the exact location of the actual activation in the brain (28, 29). In terms of the current study, the signal in the draining venules and veins may affect the BOLD fMR signal intensity in two ways.
There is increased pressure in the area adjacent to brain tumors. Radiologically this manifests as mass effect and midline shift. Venous structures are normally under low pressure and are imminently compressible. What might occur to explain the present situation is that the increased mass effect compresses the venules and larger veins, thereby speeding the egress of deoxyhemoglobin-laden blood from the area of activation. This leads to a decrease in the relative concentration of deoxyhemoglobin in the area of activation, which in turn results in an effective decrease in the difference in the concentration of deoxyhemoglobin between the resting and active states. This would lead to a decreased ability of fMR imaging to detect changes between the resting and active states (18).
In addition, brain tumors are almost invariably accompanied by an increase in blood flow. If the change in blood flow and the concentration of deoxyhemoglobin due to neural activation is the same in two patients, then the one with a relatively larger base line blood flow to the area of interest will exhibit smaller relative changes in the concentration of deoxyhemoglobin. Regarding fMR imaging, this dilution will lead to a smaller percent change in the fMR signal due to the relative dilution of the local deoxyhemoglobin concentration.
It is also possible that the venous changes adjacent to the brain tumor may actually lead to an increase in the volume on the side with the tumor revealed by BOLD fMR imaging. Patient 6 best characterizes this. The relative and adjusted activations for this patient were 4.5 and 4.64, respectively. Patient 6 was the only one to have been operated on previously. The tumor was located along the high convexity adjacent to the superior sagittal sinus. What may have occurred in patient 6 is that the previous operation partially disrupted the venous drainage from the area adjacent to the tumor, which would have led to a decrease in the venous drainage adjacent to the tumor (ie, the primary motor cortex) to accommodate an increase in venous flow. Therefore, an increase in neural activity and a corresponding increase in the blood flow to the area, coupled with a limitation on the venous drainage, led to an increase in the blood volume and deoxyhemoglobin in the vicinity of neural activation. Such a mechanism would have resulted in increased BOLD fMR signal in the vicinity of the previously operated tumor as compared with the contralateral side. It should also be noted that in patient 6 the total volume of activation at an R value of 0.45 was the smallest compared with other patients. With small numbers, a small difference in the absolute value can cause great differences in the ratio.
Contribution of Edema
It does not appear that edema in and of itself contributes significantly to the difference in the volume of activation between the two sides. For example, in patients 5 and 6, there was an actual increase in the volume of activation, notwithstanding the presence of a large volume of edema and that the edema involved the primary motor cortex itself. In patient 7, there was a very small volume of edema and the edema did not appear to involve the primary motor cortex. Nevertheless, there was a significant difference in the volume of activation between the two sides.
Effect of Tumor Size
Tumor size taken alone also does not appear to have a direct relationship to the difference in activation volume between the side with the tumor and the contralateral side. The adjusted activation for the second largest tumor (patient 4) was 1.09. It appears that, for this tumor, the difference in the relative activation was the result of tumor physically compressing the motor cortex.
General Considerations in Defining the Motor Cortex
This study demonstrated considerable variability in the pattern and volumes of activation of the motor cortices for different patients at different R values. This contention is supported by statistical analysis. The optimal R value had an average ±SD of 0.58±0.09. The range was 0.75 to 0.45. The average ±SD of the R value at which activation first occurred was 0.71±0.09. The range was 0.85 to 0.55. In patients 2, 4, 6 and 9, the optimal R value was higher than the R value at which the first activation was noted in the case of patient 7 (R = 0.55). Therefore, if one were to use an R value that was deemed optimal for patients 2, 4, 6 or 9 as the R value to define the motor cortex for patient 7, one would not see any activation. Conversely, if one used the R value deemed optimal for patient 7 to evaluate patients 2, 4, 6 or 9, there would be too much activation in areas of the brain not related to motor function to be of use in identifying the primary motor cortex.
The mean ±SD of the volume of activation at the optimal R value was 1260±412 mm3. The range was 816 to 1905 mm3. If one were to apply a single R value (eg, 0.55) to define the motor cortex for every patient, then the mean ±SD of the volume of activation would be 2228±1983 mm3, with a range of 6649 to 233 mm3. The SD for a single R value is 4.6 times as large as the SD for the optimal R value for each patient. Also the range of volumes of activation is 5.89 times larger for a single R value as opposed to the optimal R value. A large variation was also noted in the in the volume of activation at P<.05. The mean ±SD is 3009±1327 mm3.
These findings indicate that there is a significant difference in activation pattern for different patients, which are exclusive of the differences between the side with the tumor and the contralateral side. Therefore, it appears important to stress that in the preoperative analysis, to determine the location of the primary motor cortex, patients be treated individually. From the presented data, it appears that one should resist the temptation to analyze all patients by one set of statistical parameters. These data indicate that application of a uniform set of parameters would lead to overestimation in some patients and underestimation in others. It appears that different people “activate” their motor cortices differently.
Varying activation patterns in different people can be overcome in experiments that deal with large cohorts of either healthy subjects or patients with the same pathologic characteristics. In these cases, the anatomic and fMR data can be normalized to a set of generalized anatomic coordinates and the fMR data can be averaged together. Results presented in this manner allow one to make generalized statements about the type of activation one sees in certain a condition or involving a specific paradigm. This type of analysis is performed in and is adequate for the majority of experiments involving fMR imaging in such fields as cognitive neuroscience and psychology. A uniform approach, however, would not be appropriate for patients in whom actual resection of brain based on fMR data is planned. The data presented herein have demonstrated that the use of a single set of statistical parameters would lead to overestimation of the volume of the motor cortex in some patients and an underestimation in others. Such errors may lead to resection of functional cortex and postoperative neurologic deficits.
Conclusion
The results of this study demonstrate that that there is a significant difference in volumes of activation and the activation patterns of the motor cortices in different patients. Therefore, in the preoperative analysis, to determine the location of the primary motor cortex, patients should be treated individually.
In addition, this study demonstrated a significant difference in the volume of activation in the primary motor cortex between the side of the brain with the tumor and the contralateral side. This difference was most pronounced in glioblastoma multiformes. It appears that this difference may be attributable to a loss of autoregulation in the motor cortex, because of infiltration by the glioma. It also appears that changes in the pattern of venous drainage also play a role in the differences in the volume of activation of the motor cortex.
In this study, the goal of both the fMR imaging and the intraoperative physiological testing was to determine the gyrus in which the motor cortex was located. This was accomplished successfully in all patients. Therefore, the possible limitations to this method based on BOLD fMR imaging did not impede the successful identification of the motor cortex in these cases.
fig 2.
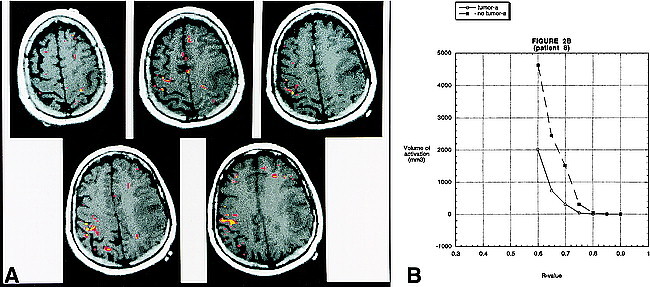
A, BOLD fMR activation from a finger-tapping paradigm coregistered to an axial T1-weighted image (500/14 [TR/TE]) in patient 8. Pathologic analysis revealed a left frontoparietal glioblastoma multiforme. The areas in yellow correspond to an R value of 0.72. The areas in red correspond to an R value of 0.67. There is robust activation in the right motor cortex. It is interesting to note that the activation is seen along the most anterior and posterior aspects of the precentral gyrus. This is the location of the cortical gray matter. The central portion of the gyrus (which contains the white matter tracts) does not reveal activation. On the right side, activation is appreciated in the postcentral gyrus, which most likely represents the sensory cortex. Activation is also identified in the left motor cortex; however, the volume is much less than on the side without the tumor. The activation on the left side is seen in the most superior and medial part of the precentral gyrus—the area relatively spared by the glioma.
B, A graph of the volume of activation for different R values for patient 8. As in figure 1, for all R values that reveal activation, the volume of activation is greater on the side opposite the tumor. The absolute volume of activation and the pattern of activation, however, differ from patient 9.
Footnotes
Supported in part by a grant from the Foundation of the University of Medicine and Dentistry of New Jersey.
Address reprint requests to Andrei I. Holodny, M.D., Department of Radiology, UMDNJ-New Jersey Medical School, University Hospital C-320, 150 Bergen Street Newark, NJ 07103-2714.
References
- 1.Maldjian JA, Schulder M, Liu WC, et al. Intraoperative functional MRI using a real-time neurosurgical navigation system. J Comput Assist Tomogr 1997;21:910-912 [DOI] [PubMed] [Google Scholar]
- 2.Schulder M, Maldjian JA, Liu WC, et al. Functional image guided surgery of intracranial tumors located in or near the sensorimotor cortex. J Neurosurg 1998;89:412-418 [DOI] [PubMed] [Google Scholar]
- 3.Rezai AR, Hund M, Kronberg E, et al. The interactive use of magnetoencephalography in stereotactic image-guided neurosurgery. Neurosurgery 1996;39:92-102 [DOI] [PubMed] [Google Scholar]
- 4.Ammirati M, Vick N, Liao Y, et al. Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery 1987;21:201-206 [DOI] [PubMed] [Google Scholar]
- 5.Devaux BC, O'Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome. J Neurosurg 1993;78:767-775 [DOI] [PubMed] [Google Scholar]
- 6.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 1957;20:22-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orrison WW, Rose DF, Hart BL, et al. Noninvasive preoperative cortical localization by magnetic source imaging. AJNR Am J Neuroradiol 1992;13:1124-1128 [PMC free article] [PubMed] [Google Scholar]
- 8.Bucholz RD. The central sulcus and surgical planning. AJNR Am J Neuroradiol 1993;14:926-927 [PMC free article] [PubMed] [Google Scholar]
- 9.Sobel DF, Gallen CC, Schwartz BJ, et al. Central sulcus localization in humans: comparison of MR anatomic and magnetoecephalographic functional methods. AJNR Am J Neuroradiol 1993;14:915-925 [PMC free article] [PubMed] [Google Scholar]
- 10.Seitz RJ, Yanxiong H, Knorr U, et al. Large-scale plasticity in the human motor cortex. Clin Neurosci Neuropathol 1995;6:742-744 [DOI] [PubMed] [Google Scholar]
- 11.Berger MS, Kincaid J, Ojemann GA, Leitich E. Brian mapping technique to maximize resection, safety and seizure control in children with brain tumors. Neurosurgery 1989;25:786-792 [DOI] [PubMed] [Google Scholar]
- 12.Cohen L, Pascual-Leone A, Hallet M. Plasticity of cortical motor output organization following deafferentation, cerebral lesion and skill acquisition. In: Devinsky O, Beric A, Dogali M, eds. Advances in Neurology: Electrical and Magnetic Stimulation of the Brain and Spinal Cord. New York, NY: Raven Press 1993;63:187-201 [PubMed] [Google Scholar]
- 13.Mogilner A, Grossman JA, Ribary U, et al. Somatosensory cortical plasticity in adult humans revealed by magnetoencephalography. Proc Natl Acad Sci USA 1993;90:3593-3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ojemann GA. Individual variability in cortical localization of language. J Neurosurg 1979;50:164-169 [DOI] [PubMed] [Google Scholar]
- 15.Ramachandran VS, Rogers-Ramachandran D, Stewart M. Perceptual correlates of massive cortical reorganization. Science 1992;258:1159-1160 [DOI] [PubMed] [Google Scholar]
- 16.Jack CR, Thompson RM, Butts RK, et al. Sensory motor cortex: correlation of presurgical mapping with functional MR imaging and invasive cortical mapping. Radiology 1994;190:85-92 [DOI] [PubMed] [Google Scholar]
- 17.Atlas SW, Howard RS, Maldjian JA, et al. Functional magnetic resonance imaging of regional brain activity in patients with intracerebral gliomas: findings and implications for clinical management. Neurosurgery 1996;38:329-338 [DOI] [PubMed] [Google Scholar]
- 18.Holodny AI, Schulder M, Liu WC, Maldjian J, Kalnin AJ. Decreased BOLD fMR activation of the motor and sensory cortices adjacent to a glioblastoma multiforme: implications for fMR guided neurosurgery. Am J Neuroradiol 1999;20:609-612 [PMC free article] [PubMed] [Google Scholar]
- 19.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets for functional MRI of the human brain. Magn Res Med 1993;30:161-173 [DOI] [PubMed] [Google Scholar]
- 20.Jack CR Jr, Thompson RM, Butts RK, et al. Sensory motor cortex: correlation of presurgical mapping with functional MR imaging and invasive cortical mapping. Radiology 1994;190:85-92 [DOI] [PubMed] [Google Scholar]
- 21.Latchaw RE, Hu X, Ugurbil K, et al. Functional magnetic resonance imaging as a management tool for cerebral arteriovenous malformations. Neurosurgery 1995;82:445-452 [DOI] [PubMed] [Google Scholar]
- 22.Fried I, Nenov VI, Ojemann SG, et al. Functional MR and PET imaging of rolandic and visual cortices for neurosurgical planning. J Neurosurg 1997;41:621-628 [DOI] [PubMed] [Google Scholar]
- 23.Rezai AR, Hund M, Kronberg E, et al. The interactive use of magnetoencephalography in stereotactic image-guided neurosurgery. Neurosurgery 1996;39:92-102 [DOI] [PubMed] [Google Scholar]
- 24.Pronin IN, Holodny AI, Kornienko VN, Petraikin AV, Golovanov AV, Lee HJ. The use of hyperventilation in contrast-enhanced MR of brain tumors. AJNR Am J Neuroradiol 1997;18:1705-1708 [PMC free article] [PubMed] [Google Scholar]
- 25.Bradac GB, Simon RS, Heidieck CH. Angiographically verified transient alteration of the intracrainial arteries and veins in dependence on different CO2 tensions. Neuroradiology 1976;10:257-262 [DOI] [PubMed] [Google Scholar]
- 26.Huber P. Functional tests in angiography of brain tumors. Neuroradiology 1970;1:132-141 [Google Scholar]
- 27.Owaga S, Lee T, Kay A, Tank D. Brain magnetic resonance imaging with contrast dependant on blood oxygenation. Proc Natl Acad Sci USA 1990;87:9868-9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boxerman JL, Bandettini PA, Kwong KK, et al. The intravascular contribution to fMRI signal change: Monte Carlo modeling and diffusion-weighted studies in vivo. Magn Res Med 1995;34:4-10 [DOI] [PubMed] [Google Scholar]
- 29.Gao JH, Miller I, Lai S, Xiong J, Fox PT. Quantitative assessment of blood inflow effects in functional MRI signals. Magn Res Med 1996;36:314-319 [DOI] [PubMed] [Google Scholar]


