Abstract
Summary: We report herein the MR finding of multiple, small, hypointense white matter foci on T1- and T2-weighted images obtained from a patient with ataxia telangiectasia. To our knowledge, this finding has not been previously reported in association with a patient with this disease entity.
Ataxia telangiectasia is a multisystem disease characterized by cerebellar ataxia, oculomucocutaneous telangiectasias, and susceptibility to certain infections and neoplastic processes. Previously reported associated MR findings of the brain include cerebellar vermian and hemispheric atrophy and diffuse increased white matter signal changes on T2-weighted images (1–3). We report a patient with long-standing ataxia telangiectasia whose T1- and T2-weighted MR images of the brain showed multiple hypointense white matter foci.
Case Report
A 31-year-old woman with a 20-year history of ataxia telangiectasia was admitted to the hospital after complaining of dizziness, headaches, and mood changes that had occurred for 2 to 3 days. The patient had a history of gait ataxia since age 4 years and was diagnosed with ataxia telangiectasia at age 7 after developing ocular telangiectasias. She initially experienced normal intellectual development, which slowed over time (she completed the second grade). Her gait and cerebellar ataxia worsened over several years, and at the time of presentation, she was confined to a wheel chair. She had a 3-year history of diet-controlled diabetes mellitus and a long-standing history of immunoglobulin A deficiency and an elevated α-fetoprotein level.
A physical examination revealed an alert and oriented young woman with moderately dysarthric speech and ocular dyspraxia with lateral and vertical gaze nystagmus. She could not walk secondary to severe ataxia and had severe finger-to-nose ataxia. She had bilateral conjunctival telangiectasias, bilateral foot drop, and no deep tendon reflex at the ankles. She had weakness of hip flexors, positive Babinski's sign bilaterally, and diminished sensation to pinprick, light touch, and vibration distal to the mid-thigh. Initial CT revealed 1.5-cm masses in the right temporal and parietal lobes. The patient's family refused further workup of these lesions, which were thought most likely to represent metastatic lesions from an unknown primary cause. MR imaging also showed cerebellar atrophy and multiple, small, nonenhancing foci of hypointensity scattered throughout the white matter of both cerebral hemispheres on conventional spin-echo T1- and T2-weighted images (Fig 1). The patient improved neurologically and was discharged 2 weeks after admission. She was readmitted 6 weeks later, with decreasing responsiveness, and died 4 days later. An autopsy was not performed.
fig 1.
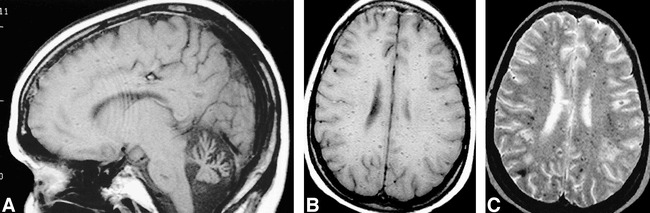
Images from the case of a 31-year-old woman with a 20-year history of ataxia telangiectasia who was admitted to the hospital after complaining of dizziness, headaches, and mood changes.
A, Spin-echo sagittal T1-weighted (600/20/1 [TR/TE/excitations]) MR image shows cerebellar atrophy.
B, Spin-echo axial T1-weighted (600/20/1) MR image shows multiple hypointense foci in the white matter bilaterally.
C, Spin-echo axial T2-weighted (2900/80/1) MR image shows multiple hypointense foci in the white matter bilaterally.
Discussion
Ataxia telangiectasia is inherited as an autosomal recessive trait with an incidence of one in 20000 to 100000 births. The disease was first named and recognized widely in a report of eight cases by Boder and Sedgwick (4). Diagnosis of the disease is made from a constellation of characteristic features, including cerebellar ataxia, oculomotor abnormalities, ocular and cutaneous telangiectasias, and immunoglobulin A, immunoglobulin E, or immunoglobulin G2 immunodeficiency, with susceptibility to sinonasal and pulmonary infections and lymphoreticular malignancies (5–8). The most commonly associated neoplasms are non-Hodgkin's lymphoma, acute lymphocytic leukemias, Hodgkin's lymphoma, and later, in young adulthood, solid tumors including breast carcinoma, gastric carcinoma, medulloblastoma, basal cell carcinoma, ovarian dysgerminoma, and hepatoma (6, 9). Patients also experience increased sensitivity to ionizing radiation, often have elevated serum α-fetoprotein levels, and may develop progeric changes (9, 10). The cause of death in 95% is malignant disease or pulmonary infection. CNS abnormalities include cerebellar ataxia, dysarthria, choreoathetosis, decreased or absent deep tendon reflexes, various oculomotor disturbances, and a mixed sensorimotor polyneuropathy. The most prominent histologic CNS finding is Purkinje cell loss (5, 7, 10).
The ataxia telangiectasia trait was mapped to chromosome 11q23 in 1988 by Gatti et al (11) and was cloned in 1995 by Savitsky et al (12). People who completely lack a functional ataxia telangiectasia mutated gene, therefore lacking the functional ataxia telangiectasia mutated protein encoded for by the gene, have ataxia telangiectasia. Studies investigating the function of the ataxia telangiectasia mutated gene have suggested its importance in telomere length, chromosomal stability, and cell cycle checkpoints (9, 13–16). Cells in patients with ataxia telangiectasia have frequent chromosomal aberrations, including chromosomal breaks, structural rearrangements, and aneuploidy. Ataxia telangiectasia cells also have subtle defects in DNA repair mechanisms. It has also been shown that several normal cell cycle checkpoints triggered after ionizing radiation exposure are lacking in ataxia telangiectasia cells, resulting in replication of damaged DNA strands. The exact relationship between these abnormal cellular activities and neuropathologic changes, such as cerebellar cell loss and atrophy, has not been elucidated.
ATM –/–mice have impaired coordination and share some histopathologic features with patients who have ataxia telangiectasia but do not show progressive cerebellar dysfunction. They also have selective dopaminergic neuron losses, a finding not present in humans with ataxia telangiectasia, but do not show widespread Purkinje cell deficiency (17, 18).
Pathologic CNS findings associated with ataxia telangiectasia include cerebellar atrophy, particularly vermian atrophy, with Purkinje, granular, and neuronal cell loss. Other reported findings include dilated cerebellar and spinal leptomeningeal veins with perivascular necrosis, dentate and olivary nuclei atrophy, and posterior column degeneration (4, 19–22). Also described are white matter “gliovascular nodules” made up of dilated capillary loops with perivascular hemorrhages and hemosiderosis, surrounded by demyelinated white matter and reactive fibrosis (23). Terplan and Krause (21) reported glial scars with dense hemosiderosis and demyelination, vascular ectasia, and “capillary angiomas.” The primary radiologic finding in patients with ataxia telangiectasia is cerebellar hemispheric and vermian atrophy (1–3). Diffuse symmetric increased T2 white matter signal has been reported in one case (1). The finding in our patient of multiple small foci of decreased white matter signal on both T1- and T2-weighted images has not, to our knowledge, been described in association with this disease. A similar appearance has been described in cases of amyloid angiopathy and disseminated intravascular coagulopathy and could also be seen with multiple cavernous angiomas (24). We postulate that these foci may represent areas of hemosiderin related to previous hemorrhage, possibly from capillary telangiectasias or the above-described gliovascular nodules. Many of the lesions appear related to the expected location of perivascular spaces, possibly indicating hemorrhage along these spaces. As suggested by Amromin et al (23), it is possible that only older patients show signs of abnormal brain vasculature, as did their patient, who was 32 years old. It may be that parenchymal vascular stigmata are present only in older patients who have ataxia telangiectasia, such as in our 31-year-old patient. To our knowledge, all reported patients who have ataxia telangiectasia and who were studied with MR imaging have been younger than 30 years.
In summary, we report a case of ataxia telangiectasia with hypointense white matter foci on T1- and T2-weighted MR images. To our knowledge, this finding has not been previously reported. These foci may reflect changes related to previous hemorrhage or abnormal white matter vasculature.
Footnotes
Address reprint requests to John J. Ciemins, MD, Department of Radiology, Resurrection Medical Center, 7435 W. Talcott Avenue, Chicago, IL 60631.
References
- 1.Sardanelli F, Parodi RC, Ottonello C, et al. Cranial MRI in ataxia-telangiectasia. Neuroradiology 1995;37:77-82 [DOI] [PubMed] [Google Scholar]
- 2.Byrd SE. Central nervous system manifestations of inherited syndromes. In: Atlas SW, ed. Magnetic Resonance Imaging of the Brain and Spine. New York: Raven 1991 562
- 3.Farina L, Uggetti C, Ottolini A, et al. Ataxia-telangiectasia: MR and CT findings. J Comput Assist Tomogr 1994;18:724-727 [PubMed] [Google Scholar]
- 4.Boder E, Sedgwick R. Ataxia-telangiectasia: a familial syndrome of cerebellar ataxia, oculocutaneous telangiectasias and frequent pulmonary infection: a preliminary report on seven children, an autopsy and a case biopsy. USC Med Bull 1957;9:15-28 [Google Scholar]
- 5.Boder E. Ataxia-telangiectasia: an overview. In: Gatti RA, Swift M, eds. Ataxia-Telangiectasia: Genetics, Neuropathology and Immunology of a Degenerative Disease of Childhood. New York: Liss 1985 1-63
- 6.Swift M, Morrell D, Cromartie E, Chamberlin AR, Skolnick MH, Bishop DT. The incidence and gene frequency of ataxia-telangiectasia in the United States. Am J Hum Genet 1986;39:573-583 [PMC free article] [PubMed] [Google Scholar]
- 7.Gatti RA, Boder E, Vinters HV, Sparkes RS, Norman A, Lange K. Ataxia-telangiectasia: an interdisciplinary approach to pathogenesis. Medicine (Baltimore) 1991;70:99-117 [PubMed] [Google Scholar]
- 8.Morrell D, Cromartie E, Swift M. Mortality and cancer incidence in 263 patients with ataxia-telangiectasia. J Natl Cancer Inst 1986;77:89-92 [PubMed] [Google Scholar]
- 9.Meyn SM. Ataxia-telangiectasia, cancer and the pathobiology of the ATM gene. Clin Genet 1999;55:289-304 [DOI] [PubMed] [Google Scholar]
- 10.Crawford TO. Ataxia telangiectasia. Semin Pediatr Neurol 1998;5:287-294 [DOI] [PubMed] [Google Scholar]
- 11.Gatti RA, Berkel I, Boder E, et al. Localization of an ataxia-telangiectasia gene to chromosome 11q22–23. Nature 1988;336:577-580 [DOI] [PubMed] [Google Scholar]
- 12.Savitsky K, Bar-Shira A, Gilad S, et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 1995;268:1749-1753 [DOI] [PubMed] [Google Scholar]
- 13.Cornforth MN, Bedford JS. On the nature of a defect in cells from individuals with ataxia-telangiectasia. Science 1985;227:1589-1591 [DOI] [PubMed] [Google Scholar]
- 14.Blocher D, Sigut D, Hannan MA. Fibroblasts from ataxia telangiectasia (AT) and AT heterozygotes show an enhanced level of residual DNA double-strand breaks after low dose-rate gamma-irradiation as assayed by pulsed field gel electrophoresis. Int J Radiat Biol 1991;60:791-802 [DOI] [PubMed] [Google Scholar]
- 15.Murray AW. Creative blocks: cell-cycle checkpoints and feedback controls. Nature 1992;359:599-604 [DOI] [PubMed] [Google Scholar]
- 16.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science 1994;266:1821-1828 [DOI] [PubMed] [Google Scholar]
- 17.Kuljis RO, Xu Y, Aguila MC, Baltimore D. Degeneration of neurons, synapses, and neuropil and glial activation in a murine Atm knockout model of ataxia-telangiectasia. Proc Natl Acad Sci U S A 1997;94:12688-12693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eilam R, Reter Y, Elson A, et al. Selective loss of dopaminergic nigro-striatal neurons in brains of Atm-deficient mice. Proc Natl Acad Sci U S A 1998;95:12653-12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguilar MJ, Kamoshita S, Landing BH, Boder E, Sedgwick RP. Pathological observations in ataxia-telangiectasia: a report of five cases. J Neuropathol Exp Neurol 1968;27:659-676 [PubMed] [Google Scholar]
- 20.De Leon GA, Grover WD, Huff DS. Neuropathologic changes in ataxia-telangiectasia. Neurology 1976;26:947-951 [DOI] [PubMed] [Google Scholar]
- 21.Terplan KL, Krauss RF. Histopathologic brain changes in association with ataxia-telangiectasia. Neurology 1969;19:446-454 [DOI] [PubMed] [Google Scholar]
- 22.Sourander P, Bonnevier JO, Olsson Y. A case of ataxia-telangiectasia with lesions in the spinal cord. Acta Neurol Scand 1966;42:354-366 [DOI] [PubMed] [Google Scholar]
- 23.Amromin GD, Boder E, Teplitz R. Ataxia-telangiectasia with a 32 year survival: a clinicopathological report. J Neuropathol Exp Neurol 1979;38:621-643 [DOI] [PubMed] [Google Scholar]
- 24.Atlas SW, Hurst RW. Intracranial vascular malfunctions and aneurysms. In: Atlas SW, ed. Magnetic Resonance Imaging of the Brain and Spine. New York: Raven 1991 521-523


