Abstract
BACKGROUND AND PURPOSE: Recent positron emission tomography and single-photon emission CT studies using the Tower of London test have shown that brain activation during planning activities primarily resides in the prefrontal cortex. In this study, we adapted the Tower of London test for functional MR imaging.
METHODS: For use with functional MR imaging, a block design of the test was created, in which planning stages were contrasted with counting of colored balls. For nine healthy participants, multisection echo-planar functional MR imaging was performed to assess brain activation based on changes in blood oxygen level. Activation maps for individual participants and a group average map were created.
RESULTS: In the group average map, activation in the dorsolateral prefrontal cortex, the anterior part of the cingulate cortex, the cuneus and precuneus, the supramarginal and angular gyrus in the parietal lobe, and the frontal opercular area of the insula was seen. These findings are in agreement with grouped data of previous positron emission tomography results. Functional MR imaging enabled us to investigate brain activation during planning activities with high spatial (and temporal) resolution in individual patients, showing that the dorsolateral prefrontal cortex was activated in all participants studied.
CONCLUSION: Presented is a working functional MR imaging version of the planning task. The high sensitivity of functional MR imaging may allow the use of this test for patients with possible (pre)frontal disorders.
Planning is defined as the ability to organize cognitive behavior in time and space (1). It is necessary in situations in which a goal must be achieved through a series of intermediate steps, each of which individually does not lead directly toward that goal. A well-known test to evaluate planning in neuropsychological research is the Tower of London test (2). For this test, the participant is instructed to move three different colored balls to match a target configuration by using a minimum number of moves. Although this test needs spatial processing abilities, it mainly depends on planning. Patients with frontal lobe pathologic abnormalities (eg, frontal lobe dementia, multiple sclerosis) perform worse than do healthy control participants.
Neuropsychological studies have shown that lesions in the frontal lobe (mainly the prefrontal cortex, which is located anterior to the motor part), might cause problems with planning (1–3). Recent positron emission tomography and single-photon emission CT studies (4–7) that used the Tower of London test confirmed that brain activity during planning is located mainly in the prefrontal area, particularly in the dorsolateral prefrontal cortex. These data were based on averages of several participants, because these techniques usually have too low a sensitivity to detect activation in individual participants reliably.
Functional MR imaging is a noninvasive technique with which to measure brain activity based on changes in the blood oxygen level (8, 9). Additional advantages of this technique compared with other functional brain imaging techniques are its high spatial and temporal resolution and the ability to study individual subjects. Paradigms applied in positron emission tomography studies, however, often cannot be used without major changes in functional MR imaging, because there are important differences in the test situations of the two techniques. In this study, the results of our fMR imaging–adapted version of the planning task are presented.
Methods
Task Paradigm
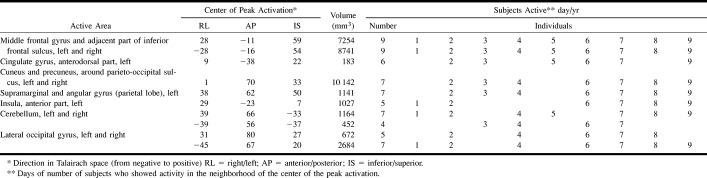
For application of the test with functional MR imaging, a block design was created, in which an “active” condition concerning planning and a “control” condition without planning were alternated (36 s per block, including an instruction; nine blocks in total). With the active condition, the participants are presented a baseline and a target configuration on a single screen (Fig 1) viewed through a mirror in the magnet bore. Both configurations consist of three balls of different colors (blue, yellow, and red) placed on three vertical rods, which are one, two, and three balls in height, respectively. The minimum number of necessary moves to reach the target has to be planned in mind. One ball can be moved at a time, and only when there is no other ball on top. Sometimes counterintuitive moves are necessary to reach the target, one of the major aspects of planning. The participant holds two air bulbs and answers by pressing the one corresponding to the side where the correct answer is shown; one of two possibilities displayed at the bottom of the screen. With the control condition, participants simply have to count the yellow and blue balls together and again choose the correct answer (total number of balls) from two possibilities. The display is almost the same as with the active condition, except that more balls are displayed, with every time another number of yellow and blue balls (Fig 1).
fig 1.
Example of the Tower of London screen.
A, Sample screen of one of the configurations of a planning problem. Upper, baseline configuration; lower, target configuration. In this example, the participant has been asked to move first the blue ball to the right rod, which is counterintuitive. Thereafter, the participant has to place the yellow ball on top of the red ball, the blue ball at its destination, the yellow ball on top of the blue ball, the red ball on the right rod, and, finally, the yellow ball at the target position (sixth move). Two alternatives are presented on each side of the screen, from which the participant had to choose the correct answer. The participant was asked to respond by pressing the air bulb at the corresponding side.
B, Sample screen of the control configuration. The participant has to count the yellow and blue balls altogether. In this example, the answer is six, which is indicated on the right.
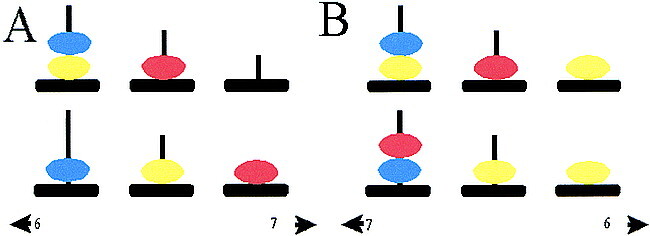
Every condition block starts with an instruction of 4 s to plan the moves (active condition) or count the balls (control condition). Easy (two to four moves) and difficult (five to seven moves) configurations are presented in separate blocks to make it possible to compare two different levels of planning activity (easy and difficult) and a control situation without planning. The whole test is self-paced; a new trial (in the same block) is presented only after a response is obtained. No feedback regarding the correctness of the answer is provided during the task. After 36 s, the next block starts with a new instruction (Fig 2), regardless of whether there was a response to the last trial. In total, 82 whole-brain volumes (nine blocks with nine volumes each and one volume preceding the start of the test) were scanned (one scan was obtained every 4 s) (Fig 2). To ensure the participants were familiar with the procedure, the test was explained and practiced outside the procedure room before MR imaging was performed.
fig 2.
Overview of the test, imaging, and data analysis. With this task paradigm, easy and difficult planning and counting are performed in blocks. Every block lasts 36 s, including a 4-s instruction. A total of nine blocks were performed during the test. A total of 82 images were obtained, including those of a dummy before the test started. To account for the hemodynamic response delay, a 4-s delay in the analysis was used. The images obtained during the instruction (accounting for the hemodynamic response delay) were not used for further calculations. The resulting 71 images were used for the analysis (24 obtained during the difficult planning problems, 24 obtained during the easy planning problems, and 23 obtained during the control stage)
Participants
Nine healthy students (five men and four women; mean age, 22 years; age range, 20–27 years) were evaluated. The ethical review board of the Academic Hospital of the Vrije Universiteit Amsterdam approved of the study, and all participants provided informed consent.
Data Acquisition
Imaging was performed on a 1.5-T MR system with a standard circularly polarized head coil. Anatomic imaging was performed with a 3D gradient-echo T1-weighted sequence (15/7/1 [TR/TE/excitations]; flip angle, 8°; matrix, 256 × 256; field of view, 220 × 220 mm; section thickness, 2 mm; number of sections, 82). The sections were planned in the coronal plane with a rotation of approximately 30° of the cranial part in the anterior direction, to cover the whole brain in the least possible number of sections. For functional MR imaging, a whole-brain echo-planar imaging sequence (4000/64/1; flip angle, 90°; matrix, 64 × 128 interpolated to 128 × 128 mm; field of view, 220 × 220 cm; section thickness, 6 mm, intersection gap, 1.02 mm; number of sections, 23) was used. The echo-planar imaging sections were planned parallel to the anatomic sections.
Data Analysis
The first step of postprocessing was correction of motion artifacts (10), with the consequence of corrupting the first and last section of each volume, which were discarded from further analysis. Next, the data were smoothed in-plane, resulting in a full width at half maximum of 5 mm in plane. The following steps were performed with AFNI software (11). Activation was detected by correlating the time course of each voxel with a box car function representing the active and control blocks of the paradigm (Fig 2). Also, the two levels of planning difficulty were correlated in the same way, without using the control stage images. Voxels with a signal increase during the active condition had a positive correlation coefficient and were called positive activation. The opposite was true for voxels with a relatively reduced signal. The box car function was delayed 4 s (1 image) in time to account partly for the hemodynamic response delay (12). The images that corresponded with the time the instructions were displayed were not used in the analysis (Fig 2).
The images were transformed into Talairach coordinate space (13) by defining reference line landmarks on the anatomic images. Individual activation maps were calculated and also used to create a group average. Correction for multiple comparisons was performed, accounting for the spatial extent of activation (14, 15). Only voxels with a P value of at least 10−4 were considered active, and 3D clusters of at least 104 mm3 (ie, five connected active voxels) were included, resulting in a mean activation map of all participants with an overall P value < .05. In all individual and group images, the macroscopic position of significant activations was defined based on the visible gyral-sulcal pattern to specify the location in more anatomic detail.
Results
All nine participants were studied successfully. Because we used a self-paced paradigm, the number of answers varied, with a mean of 13.7 answers (range, 9–17 answers) during both planning conditions together, 78.7% of which were correct (66.7–100%). For the easy configurations, a mean of 8.5 answers (6–11 answers) was provided, 82% of which (57–100%) were correct; for the difficult configurations, the results were 5.2 answers (3–8 answers), 71% of which (50–100%) were correct. With the control condition (only counting), the number of correct answers was 98% (89–100%), and the participants gave 36.4 answers (29–48 answers).
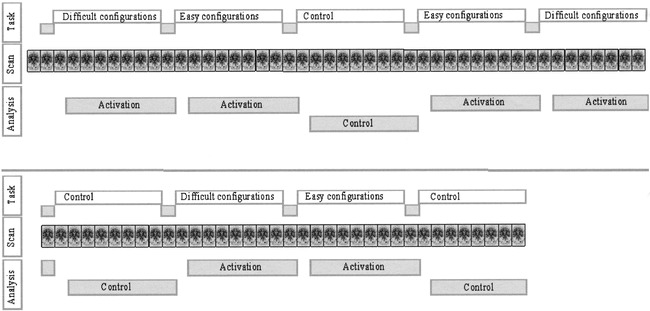
Activation in the group average images during the active condition (easy and difficult configurations combined) was seen on both sides in the frontal and parietal lobes, the cerebellum, and the insula (Fig 3 and Table). The frontal area showed activation bilaterally in the middle frontal gyrus and the adjacent part of the inferior frontal sulcus (with some preference for the right hemisphere) and in the anterior part of the cingulate gyrus (Fig 3A). The parietal and occipital regions involved were the precuneus and cuneus and the left supramarginal and angular gyrus (Fig 3B).
fig 3.
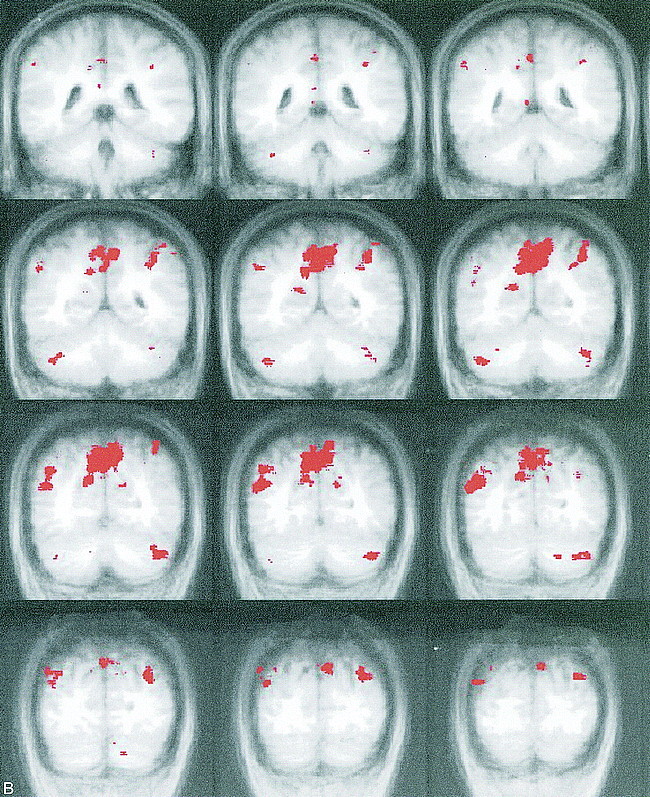
Activated areas during the active condition of the Tower of London task. During the active condition (planning stage) of the task, activation (red) on the group average map (shown in Talairach format with coronal orientation) is shown. In the brain area from −3 anterior to +49 posterior, no activation was seen. A, Frontal regions. Coronal sections of coordinates −44 to −3 (anterior part of the brain). Activity was noted in the dorsolateral prefrontal cortex, the anterior part of the cingulate cortex, a part of the precentral cortex, and the frontal opercular area of the insula during planning. The right side shows slightly more activation than the left side. B, Parietal/occipital regions. Coronal sections of coordinates +49 to +80 (posterior part of the brain). Activation was noted in the cuneus and precuneus region, the marginal and angular gyrus in the parietal lobe, and the cerebellum during the active condition
Areas of fMR activation during the Tower of London task; planning condition (mean of all subjects)
Most participants showed activation in the same gyri that were activated on the group average (Table), and activation in the middle frontal gyrus (bilaterally) especially occurred in all participants. We did not find any significant differences in the activation when comparing the two levels of difficulty (easy and difficult planning).
The areas observed with a higher signal with the control condition were the middle part of the cingulate gyrus and the middle part of the insula on both sides, the fusiform gyrus and the pre- and postcentral gyrus. The majority of cases (67–100%) showed activation in the same gyri that were activated on the group average. An exception was the activation in the fusiform gyrus, which had a higher signal with the control condition compared with the planning condition, in only 44% of the participants (Fig 3 and Table).
Discussion
A planning task, such as the Tower of London, has proven to be sensitive to prefrontal lesions (3, 5, 16). A new version of the planning task, adapted for functional MR imaging, is presented. The group analysis showed activation during the planning stages in the dorsolateral prefrontal cortex, the anterior part of the cingulate cortex, the cuneus and precuneus, the supramarginal and angular gyrus in the parietal lobe, and the frontal opercular area of the insula. These findings are in full agreement with grouped data of previous positron emission tomography results (5–7). In addition, functional MR imaging enabled us to investigate brain activation during planning activities with high spatial (and temporal) resolution in individual participants, showing that the dorsolateral prefrontal cortex was activated in all individual participants studied.
Previous studies have indicated that activation associated with this task performance occurs especially in the prefrontal cortex (1, 2, 4–7). Three positron emission tomography studies that evaluated the Tower of London test with healthy control participants (5–7) are herein discussed in comparison with our results.
The positron emission tomography studies conducted by Owen et al (5), Baker et al (6), and Dagher et al (7) used a block paradigm in which the participants had to plan the moves and press on a touch screen the number of moves (6) or perform each move separately by pressing on a touch screen the ball that has to be moved and thereafter the place to which it had to be moved (5, 7). With the control condition, the participants did not need planning but only had to view the subsequent moves (6) or press the touch screen at the highlighted locations corresponding with locations pressed during the planning condition (5, 7). Dagher et al not only analyzed the activation during planning, but the planning stages were analyzed also in a parametric way, based on task complexity (7).
The three aforementioned positron emission tomography studies (group analysis) showed frontal lobe activation, in the dorsolateral prefrontal cortex bilaterally (predominantly right hemisphere), the anterior cingulate gyrus bilaterally, and some frontal lobe motor areas. Activation was also noted in other frontal areas but not with complete consistency across the three studies; Owen et al (5) activated the left medial frontal cortex, and Baker et al (6) activated the right rostrolateral prefrontal cortex. All three studies also showed activation in the caudate nuclei (the left one in the study conducted by Baker et al and the right one in the other two studies). In addition to the frontally located activated areas, activation was also seen in the right anterior insula (frontal opercular area), the medial parietal cortex (precuneus) bilaterally, the left inferior parietal cortex, the right superior parietal cortex bilaterally, the lateral occipital cortex, and the left cerebellum and vermis (5–7). Those areas are probably activated not only by the planning process itself but also by the motor and visual processes needed to perform this planning.
In the study conducted by Dagher et al (7), the activated areas during planning could be divided, as a result of the parametric analysis, into those that did not correlate with the task complexity, such as the areas belonging to the dorsal stream of visual input (visual and posterior parietal cortical areas) and the execution of arm movements (frontal lobe motor areas) and those that correlated with the task complexity, such as lateral premotor cortex, rostral anterior cingulate cortex, dorsolateral prefrontal cortex bilaterally, and the right dorsal caudate nucleus.
In our functional MR imaging study, we found globally the same activated areas as in the aforementioned positron emission tomography studies (Table). Concerning the frontal areas, we observed significant activation in the middle frontal gyrus and the adjacent part of the inferior frontal sulcus, the precentral cortex, and the anterior part of the cingulate gyri. As in the positron emission tomography studies, activation was also noted in the caudate nuclei, but the volume of this activation was below our cluster size limit. We found only one main difference with the positron emission tomography studies. In our experiment, the occipital lobe and the primary motor areas were more active during the control condition. This could be explained in that with the control condition, the total number of configurations processed was higher.
One of the main areas activated during this planning task is the dorsolateral prefrontal cortex. Activation in this area is thought to be associated with active processing of both spatial and nonspatial information. Left-right differences probably exist, but there is no consensus regarding the nature of those differences. Baker et al (6) refer to literature on positron emission tomography in which the spatial information is predominantly represented in the dorsolateral prefrontal cortex of the right hemisphere, whereas nonspatial working memory should be positioned predominantly at the left side. In our study, we noted bilateral frontal activation with a slightly larger activated area on the right side. This may be taken to indicate that spatial information processing is a prominent feature of the Tower of London paradigm. Such a suggestion seems logical in view of the test; moreover, activation of the precuneus and inferior parietal lobe has been associated with spatial processes and is correlated with prefrontal activity (6, 7).
In contrast with the study conducted by Dagher et al (7), who found complexity-related activation when performing a parametric analysis on five difficulty levels, including one-move problems (which require almost no planning), no differences in activation were found in our study when comparing the easy and difficult conditions. This could be explained by the decreased amount of data, our design of only two levels, and too small a difference of the two levels of planning. Another possible explanation is the difference in the response to be provided: in our test, one number, and in the study conducted by Dagher et al, the whole planning sequence, the last of which probably requires more planning activities.
Regarding the interpretation of the test score, for all except one participant, the test score was clearly beyond chance expectations (ie, more than 50% correct answers). The test score is of assistance only in determining whether the participant has performed the test or when no activation or activation in unexpected areas is seen, which was not the case for our participants. With the non-imaging versions of the test, the reliability of the test scores within individual participants is sometimes criticized. Our study tried only to localize the underlying brain areas activated, so the test score, and thereby its within-participants reliability, was less important for our goal. The high intersubject concordance for prefrontal activation may relate to the fact that for this test, it is not the number of correct answers but the process of planning (resulting in a correct answer or not) that is the most important determinant.
One of the main ideas regarding the use of functional MR imaging was to study participants individually (Table). All nine participants tested showed significant activation in the dorsolateral prefrontal cortex when analyzed individually. The other areas that showed activation in the group analysis were also seen in most participants. Future research with functional MR imaging will enable us to correlate those findings with individual parameters.
Conclusion
The Tower of London test was successfully adapted for functional MR imaging, and the activated areas found were consistent with those of previous positron emission tomography studies, especially in the prefrontal cortex. Also, functional MR imaging allowed us to show significant activation in individual participants. The dorsolateral prefrontal cortex was active in all individual participants. The benefits of the functional MR imaging procedure could enable us to use this adapted version of this test to evaluate individual patients with presumed prefrontal dysfunctions.
Acknowledgments
The Dutch MR Centre for MS Research is supported by the Stichting Vrienden MS Research, the University Hospital Vrije Universiteit, and the Medical Faculty of the Vrije Universiteit.
Footnotes
This work was supported in part by grants 96-278 and 97-330 from Stichting Vrienden MS Research (to R.H.C.L.).
Address reprint requests to R.H.C. Lazeron, Academic Hospital of the Vrije Universiteit, Department of Neurology, P.O. Box 7057, 1007 MB Amsterdam, The Netherlands.
References
- 1.Owen AM. Cognitive planning in humans: neuropsychologicalal, neuroanatomical and neuropharmacological perspectives. Prog Neurobiol 1997;53:431-450 [DOI] [PubMed] [Google Scholar]
- 2. Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci 1982;298:199-209 [DOI] [PubMed] [Google Scholar]
- 3.Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain 1991;114:727-741 [DOI] [PubMed] [Google Scholar]
- 4.Morris RG, Ahmed S, Syed GM, Toone BK. Neural correlates of planning ability: frontal lobe activation during the Tower of London test. Neuropsychologia 1993;31:1367-1378 [DOI] [PubMed] [Google Scholar]
- 5.Owen AM, Doyon J, Petrides M, Evans AC. Planning and spatial working memory: a positron emission tomography study in humans. Eur J Neurosci 1996;8:353-364 [DOI] [PubMed] [Google Scholar]
- 6.Baker SC, Rogers RD, Owen AM, et al. Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia 1996;34:515-526 [DOI] [PubMed] [Google Scholar]
- 7.Dagher A, Owen AM, Boecker H, Brooks DJ. Mapping the network for planning: a correlation PET activation study with the Tower of London task. Brain 1999;122:1973-1987 [DOI] [PubMed] [Google Scholar]
- 8.Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med 1990;14:68-78 [DOI] [PubMed] [Google Scholar]
- 9.Kwong KK, Belliveau JW, Chesler DA, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A 1992;89:5675-5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr 1992;16:620-633 [DOI] [PubMed] [Google Scholar]
- 11.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162-173 [DOI] [PubMed] [Google Scholar]
- 12.Kim SG, Ugurbil K. Functional magnetic resonance imaging of the human brain. J Neurosci Methods 1997;74:229-243 [DOI] [PubMed] [Google Scholar]
- 13.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme Verlag 1988;
- 14.Cox RW, Jesmanowicz A, Hyde JS. Real-time functional magnetic resonance imaging. Magn Reson Med 1995;33:230-236 [DOI] [PubMed] [Google Scholar]
- 15.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995;33:636-647 [DOI] [PubMed] [Google Scholar]
- 16.Rezai K, Andreasen NC, Alliger R, Cohen G, Swayze V, O'Leary DS. The neuropsychology of the prefrontal cortex. Arch Neurol 1993;50:636-642 [DOI] [PubMed] [Google Scholar]