Abstract
BACKGROUND AND PURPOSE: Anatomic evaluation of the lenticulostriate arteries (LSAs) is important, especially in cases of surgical or endovascular treatment, but biplanar angiography provides limited information. We analyzed the LSAs with rotational angiography (RA) and 3D reconstruction and compared the findings with those of previous autopsy studies.
METHODS: We retrospectively reviewed the LSAs on 3D reconstructed RA images (200 sides in 159 cases) to analyze their origin, course, and supplying territories. The angiographic configurations of the LSAs were classified by using the scheme devised by Yasargil.
RESULTS: LSAs from the anterior cerebral artery were demonstrated on 140 sides (70%). They originated from the A1 segment in 59 sides, from the A1-A2 junction on 41 sides, and from the A2 segment on 35 sides. They arose as a single trunk and ended as the most medial and anterior perforators in most cases. Arteries from the middle cerebral artery (MCA) were demonstrated on 190 sides (95%). They originated from the M1 segment on 131 sides, from the MCA bifurcation on 26 sides, and from the M2 segment on 33 sides; notably, all LSAs arising from the M2 segment originated from the superior division. In terms of configuration, we identified four major and 11 minor types. The most frequent LSA type was the combination of medial and lateral distal striate arteries, which comprised 53 sides (28%).
CONCLUSION: Analysis of LSAs with RA and 3D reconstructed images may provide useful information in patients with cerebrovascular diseases, especially before surgical and endovascular treatment.
Anatomic studies of the lenticulostriate arteries (LSAs) have been described in the literature (1–10). Evaluation of the anatomy of the LSAs is important, especially in cases in which surgical or endovascular intervention is planned. However, to our knowledge, no study has been conducted to accurately document the LSAs in living patients before they undergo interventional procedures.
With simultaneous rotation of the X-ray tube and image intensifier during the intra-arterial injection of contrast medium, rotational angiography (RA) and 3D reconstructed images enable us to obtain accurate information regarding the cerebral arteries and their branches. This technique provides images from various points of view, thereby avoiding overlapping of the vessels (11–15). The purpose of our study was to evaluate the application of RA and 3D reconstructed images to the angiographic study of the LSAs.
Methods
Angiographic findings in 159 patients (64 men, 95 women) were retrospectively reviewed. The patients’ mean age was 49 years, with a range of 14–84 years. The cerebral arteries were evaluated on the right side in 98 patients and on the left side in 102, for a total of 200 sides. The most common angiographic diagnosis was aneurysm (Table 1). Also included were 14 sides in 13 patients with an accessory or duplicated middle cerebral artery (MCA).
TABLE 1:
Angiographic diagnoses in 159 cases (200 sides)
| Diagnosis | No. of Sides |
|---|---|
| Aneurysm | 110 |
| Stenosis | 3 |
| Fenestration | 14 |
| Accessory/duplicated MCA | 14 |
| Median callosal artery | 2 |
| AICA from AchA* | 1 |
| Negative | 70 |
Anterior inferior cerebellar artery from the anterior choroidal artery.
Angiographic examinations were performed by using a commercially available biplanar angiographic unit (Integris Allura; Philips Medical Systems, Best, the Netherlands) with an image intensifier matrix of 1024 × 1024. The C-arm rotated over a 240° range at a rate of 55°/s for about 4.4 seconds. The contrast medium was injected at a flow rate of 4–5 mL/s, resulting in a total of 16–20 mL for each RA acquisition. The image data were transferred to a workstation, where reconstruction of 3D images by volume rendering was performed with a software package (Integris 3D-RA release 3.2; Philips Medical Systems). Choosing an adequate threshold was a prerequisite for accurately demonstrating the LSAs, for reducing artifacts from adjacent bone, and for avoiding fusion of objects (Fig 1). We analyzed snapshots consisting of six basic views (anterior, posterior, bilateral, superior, and inferior views), in addition to views of arbitrary angles to comprehensively demonstrate the origin and course of the LSAs.
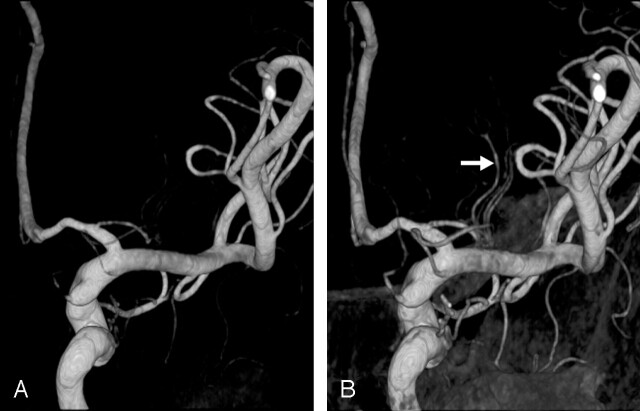
Fig 1.
Choosing an adequate threshold is important for demonstrating the LSAs clearly. Posterior images of the right ICA.
A, LSA stump arising at the proximal A1 region.
B, An adequate threshold is selected, and three branches (arrow) stemming from an LSA trunk are clearly depicted.
We analyzed the origin, course, and supplying territories of the LSAs. The LSAs were classified into one of the following groups, according to their origin: 1) medial distal striate (MDS), also known as the recurrent artery of Heubner, long central artery, or telencephalic artery; 2) medial proximal striate (MPS), also known as the short central artery, diencephalic artery, or medial LSA; 3) lateral proximal striate (LPS); or 4) lateral distal striate (LDS) group (16). The LPS and LDS groups were commonly called the lateral LSAs, collectively.
Results
LSAs and Their Classification
The origins of the lateral LSAs were best observed on the posterior views; those of the medial LSAs were best observed on superior views (Figs 2–5). To evaluate the LSA destinations, superior and lateral views were helpful (Figs 2 and 3). The LSAs from the anterior cerebral artery (ACA) (medial LSAs) were demonstrated on 140 sides (70%). They originated from the A1 segment on 59 sides, from the A1-A2 junction on 41 sides, from the A2 segment on 35 sides, and from the anterior communicating artery on five sides. They arose as a single trunk and ended as the most medial and anterior perforator in most cases. Typically, the single LSA from the ACA (especially the A2 segment) corresponding to the recurrent artery of Heubner, ran a recurrent course along the A1 segment in the anterior and inferior direction and then crossed the A1 segment in the posterior and superior direction, passing the posterior aspect of the bifurcation of the internal carotid artery (ICA) and ending as perforators.
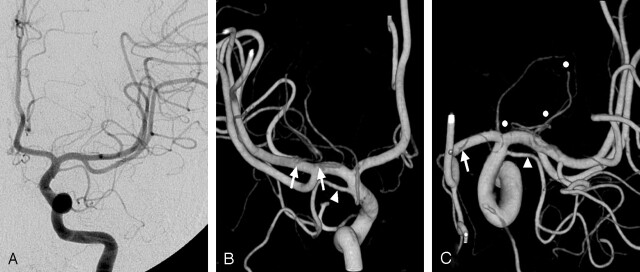
Fig 2.
Origins of the LSAs.
A, On the anteroposterior left ICA angiogram, exact origins of the LSAs cannot be evaluated.
B, On the posterior 3D reconstructed image, separate origins of the lateral LSAs are well delineated (arrows). A duplicated MCA (arrowhead in B and C) arises just proximal to the ICA bifurcation, giving cortical branches to anterior temporal lobe. No perforators from the duplicated MCA can be identified.
C, On the superior view, destinations of the LSAs (circles) and the origin of a medial LSA (arrow) are well demonstrated. LSAs with a more lateral origin supply the more posterior areas of the central brain.
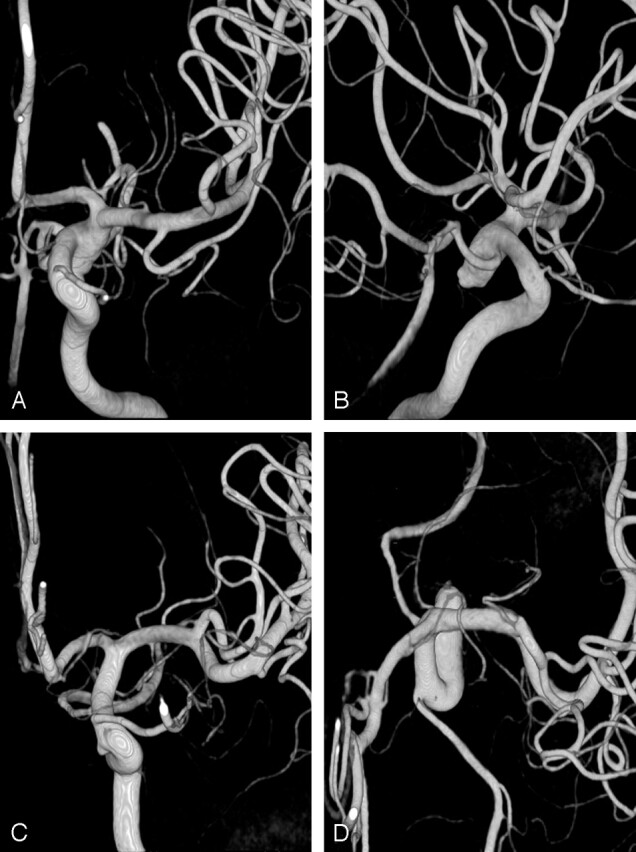
Fig 5.
Configurations of accessory MCAs and LSAs.
A, Accessory MCA of distal origin arising from the A2 segment of the ACA gives off an LSA (arrow).
B, Accessory MCA of proximal origin arising from proximal A1 segment of the ACA gives off LSAs (arrow).
C, Accessory MCA of distal origin gives off a recurrent artery of Heubner (arrow). A separate LSA (arrowhead) arises from the MCA.
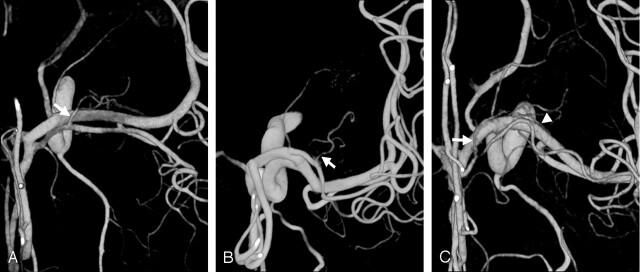
Fig 3.
Direction of LSAs.
A and B, Anterior (A) and medial (B) images. More medially located LSA (arrow) divides into two branches and has the more anterior direction of supply. More lateral LSA (arrowhead) arises as a single trunk and has the more posterior direction of supply.
C and D, Anterior (C) and superior (D) images. LSA arising from the A1-A2 junction (recurrent artery of Heubner, arrow) is directed anteriorly on the superior view. LSA arising from the superior division of the MCA (arrowhead) is directed posteriorly.
The LSAs from the MCA (lateral LSAs) were demonstrated on 190 sides (95%). They originated from the M1 segment on 131 sides, from the MCA bifurcation on 26 sides, and from the M2 segment on 33 sides; notably, all the LSAs arising from the M2 segment originated from the superior division. They arose as a single trunk on 107 sides, as two trunks of a common origin on 56 sides, and as three trunks of a common origin on 13 sides. They ended as the intermediate and lateral groups of perforators, each group supplying the middle and posterior part of the basal ganglia. Therefore, the LSAs of more medial origin supplied the more anterior parts of the basal ganglia, while the LSAs of more lateral origin supplied the more posterior parts of the basal ganglia (Figs 2 and 3). When they were multiple, the orifices of the lateral LSAs supplying the more lateral (therefore, posterior) part of the central brain tended to be located at more posterior aspects of the MCAs.
When we applied the Yasargil classification of LSAs (16), the LDS group was identified most frequently, followed in order by the MDS, LPS, and MPS groups (Table 2). We could therefore classify the configuration of LSAs according to the combinations of groups of LSAs on 186 sides, excluding cases of an accessory or duplicated MCA (Table 3). We were able to find all 15 possible combinations of the four groups of LSAs. (No case was without LSAs.) The MDS-LDS configuration was the most frequent (n = 53, 28%) followed in order by the LPS-only (n = 29, 15%), MDS-LPS (n = 27, 15%), and LDS-only (n = 22, 12%) configurations. The other minor combinations accounted for the remaining 55 sides (30%). Figure 4 shows several examples.
TABLE 2:
Identification of LSAs on 3D reconstructed images on 186
| Group | No. of Sides (%) |
|---|---|
| MDS | 101 (54) |
| MPS | 35 (19) |
| LPS | 86 (46) |
| LDS | 109 (59) |
Note.—The LDS group was the most frequently identified. Cases of accessory or duplicated MCAs (n = 14) were excluded.
TABLE 3:
Combinations of LSAs on 3D reconstructed images on 186 sides
| Combination | Group |
No. (%) | |||
|---|---|---|---|---|---|
| MDS | MPS | LPS | LDS | ||
| Major | |||||
| 1 | Y | N | N | Y | 53 (28) |
| 2 | N | N | Y | N | 29 (15) |
| 3 | Y | N | Y | N | 27 (15) |
| 4 | N | N | N | Y | 22 (12) |
| Minor | |||||
| 1 | N | Y | N | Y | 10 (5) |
| 2 | N | Y | Y | N | 10 (5) |
| 3 | N | N | Y | Y | 9 (5) |
| 4 | Y | N | Y | Y | 8 (4) |
| 5 | N | Y | N | N | 4 (2) |
| 6 | Y | N | N | N | 4 (2) |
| 7 | Y | Y | N | Y | 3 (2) |
| 8 | N | Y | Y | Y | 2 (1) |
| 9 | Y | Y | Y | N | 2 (1) |
| 10 | Y | Y | N | N | 2 (1) |
| 11 | Y | Y | Y | Y | 1 (1) |
Note.—The four major combinations accounted for 70% of all cases. Cases of accessory or duplicated MCAs (n = 14) were excluded.
Fig 4.
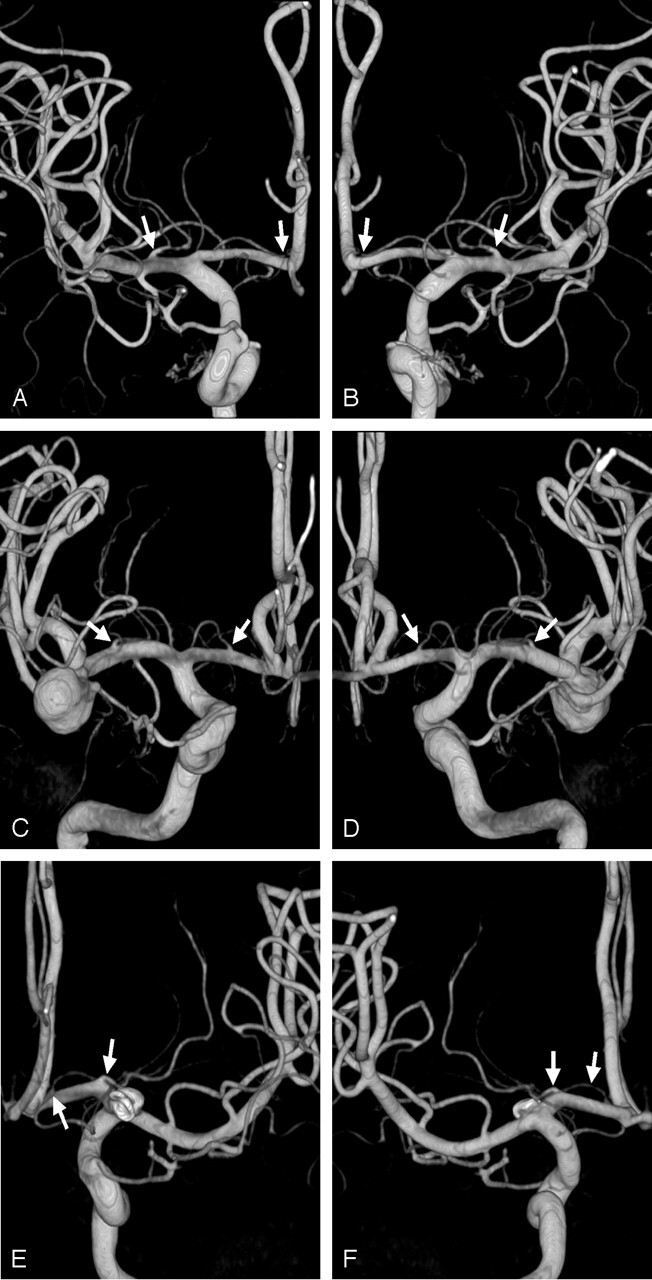
Examples of LSA configurations. Arrows indicate the origins of LSAs.
A and B, Posterior (A) and anterior (B) images of MDS/LPS configuration (n = 27, 15%).
C and D, Anterior (C) and posterior (D) images of MPS/LPS configuration (n = 10, 5%).
E and F, Posterior (E) and anterior (F) images of MDS/MPS configuration (n = 2, 1%).
LSAs in Cases of Accessory or Duplicated MCAs
Eleven accessory MCAs arising from the ACA and three duplicated MCA arising from the ICA (17) were included in our series. One patient had an accessory MCA on the left side and a duplicated MCA on the right. We found no contribution to the LSA from the duplicated MCAs (Fig 2). In contrast, we found LSAs originating from the accessory MCA in all cases but one. In five cases with an accessory MCA, this was the only provider to the LSAs; in the other five cases, LSAs of separate origins from the ACA or MCA were identified, as well as LSAs from accessory MCA. A few examples are shown in Figure 5.
Discussion
The diagnostic value and applicability of RA in defining aneurysms and stenoses was initially reported during the 1970s (18, 19). RA enables an accurate evaluation of an artery by providing images from various points of view; these are achieved with simultaneous rotation of the X-ray tube and the image intensifier during an intra-arterial injection of contrast medium. Its applications to the evaluation of carotid disease are already well known (20–22). Since the development of the 3D reconstruction technique during the 1990s (23, 24), the applications of RA with 3D reconstruction have been prolifically reported in the field of cerebral aneurysms (11–15) and cerebral arteriovenous malformations (25). However, to our knowledge, ours is the first angiographic study of the LSAs based on an RA and 3D reconstruction technique.
Understanding of the LSAs
Earlier studies of LSAs involved cadaver brains (1–10) and focused on the microvascular relationships important for the surgical treatment of aneurysms in the anterior communicating region; these included studies of the recurrent artery of Heubner (1–3, 7, 10). They tried to overcome difficulties in visualizing the anterior communicating artery and adjacent vessels, which are frequently overlapping and hard to delineate on the basis of angiographic findings. Previous research has provided valuable data regarding the fine anatomy of the LSAs. Despite these contributions, it is not easy to apply this information to a particular case before surgical or endovascular intervention, because the LSAs have so many variations and because surgeons can confirm the LSA anatomy only after time-consuming and meticulous dissection under the microscope in the operating theater.
Our study demonstrates that accurate information about the LSAs can be obtained by merely looking at the 3D reconstructed RA images. Four major configurations of the LSAs covered 70% of all cases. However, 11 minor configurations of the LSAs were also observed. The supply to the central brain seems to maintain a balance between medial LSAs and lateral LSAs: When the lateral LSAs are prominent, there is little supply from medial LSAs, and vice versa. In this study, we demonstrated that any particular combination of LSAs can be documented prior to intervention by using the RA and 3D reconstruction technique. A detailed understanding of LSA anatomy may be important, because unexpected damage to the LSA can cause various clinical syndromes owing to ganglionic-capsular infarction (6). With this information, we can be more careful and thus avoid damaging the LSAs during interventional procedures, especially in cases of cerebral fusiform and saccular aneurysms and in cases of cerebral arterial stenoses managed with angioplasty with or without stent placement. It is important to know about the four major and 11 minor configurations, as presented in our study; however, it is more important to know what LSA configuration a particular case has in advance of any interventions. The RA and 3D reconstruction technique can provide this information.
Categorization of the LSA configuration seems to be meaningful for analyzing them. In this study, we used the Yasargil classification because it is inclusive, systematic, and easy to apply in our analysis of the images (16). Also, perfusion of the central brain is balanced among the various sources of vascular supply, and knowledge about the parts of the main cerebral arteries that are contributing to the LSAs in a particular case is useful. An analysis based on this categorization may possibly be applied to cases involving infarction in the central brain; in this way, we might be able to document the cause of the ischemic changes at the LSA level.
Understanding of Accessory or Duplicated MCAs
Our series included 13 cases (14 sides) of accessory or duplicated MCAs.
According to our findings, accessory MCAs assist in supplying the central brain by giving off LSAs, whereas duplicated MCAs give off only cortical branches, as Takahashi et al (26) previously described. Because biplanar angiography provides limited visual angles, it is limited in accurately documenting the origin of the LSAs—that is, whether they are from accessory MCAs or from the main trunk of the MCA. We could accurately document the origins of the LSAs in cases of accessory MCAs by using RA and 3D reconstructed images from various angles, thereby avoiding overlapping of the vessels. The LSAs arise anywhere along the accessory MCAs, as shown in Figure 5. We speculate that accessory MCAs are entities within a spectrum of early frontal branches of the MCA, rather than just variants of a recurrent artery of Heubner (26, 27). In an anatomic study, Tanriover et al (8) reported that LSAs arose from 81% of the early frontal branches. When we consider a situation in which the early frontal branches arise from the ACAs, they are not distinguishable from the accessory MCAs. Blood supply to the central brain can possibly be taken from the ACA dominantly or from the MCA dominantly during embryonic development. Likewise, blood supply to the lateral frontal lobe may have an origin from the ACA; in this case, the artery becomes an accessory MCA.
Study Limitations
Our study has a limitation in that we could not document the exact site of penetration of the vessels through the anterior perforating substance, as shown in a report by Rosner et al (28), because our study was basically an angiographic study and not one showing the brain parenchyma. Despite this limitation and in light of previous cadaveric studies (1–10), the fine vessels arising from the superoposterior aspects of the proximal ACAs and MCAs, as visualized by using the RA and 3D reconstruction technique, are LSAs. In addition, the course and direction of the supply of the vessels shows us they are perfusing the central brain and not the cortices.
Conclusion
RA with 3D reconstruction is a valuable technique for accurately defining the angiographic anatomy of the LSAs. It can thereby provide important information before surgical and endovascular treatment.
References
- 1.Gomes F, Dujovny M, Umansky F, et al. Microsurgical anatomy of the recurrent artery of Heubner. J Neurosurg 1984;60:130–139 [DOI] [PubMed] [Google Scholar]
- 2.Gomes FB, Dujovny M, Umansky F, et al. Microanatomy of the anterior cerebral artery. Surg Neurol 1986;26:129–141 [DOI] [PubMed] [Google Scholar]
- 3.Gorczyca W, Mohr G. Microvascular anatomy of Heubner’s recurrent artery. Neurol Res 1987;9:259–264 [DOI] [PubMed] [Google Scholar]
- 4.Marinkovic SV, Kovacevic MS, Marinkovic JM. Perforating branches of the middle cerebral artery: microsurgical anatomy of their extracerebral segments. J Neurosurg 1985;63:266–271 [DOI] [PubMed] [Google Scholar]
- 5.Marinkovic S, Gibo H, Milisavljevic M. The surgical anatomy of the relationships between the perforating and the leptomeningeal arteries. Neurosurgery 1996;39:72–83 [DOI] [PubMed] [Google Scholar]
- 6.Marinkovic S, Gibo H, Milisavljevic M, Cetkovic M. Anatomic and clinical correlations of the lenticulostriate arteries. Clin Anat 2001;14:190–195 [DOI] [PubMed] [Google Scholar]
- 7.Permutter D, Rhoton AL Jr. Microsurgical anatomy of the anterior cerebral-anterior communicating-recurrent artery complex. J Neurosurg 1976;45:259–272 [DOI] [PubMed] [Google Scholar]
- 8.Tanriover N, Kawashima M, Rhoton AL Jr, Ulm AJ, Mericle RA. Microsurgical anatomy of the early branches of the middle cerebral artery: morphometric analysis and classification with angiographic correlation. J Neurosurg 2003;98:1277–1290 [DOI] [PubMed] [Google Scholar]
- 9.Umansky F, Gomes FB, Dujovny M, et al. The perforating branches of the middle cerebral artery. J Neurosurg 1985;62:261–268 [DOI] [PubMed] [Google Scholar]
- 10.Vasquez-Loayza M, Dujovny M, Agner C, Misra M. Microsurgical anatomy of the short central artery. Neurol Res 1998;20:209–217 [DOI] [PubMed] [Google Scholar]
- 11.Abe T, Hirohata M, Tanaka N, et al. Clinical benefits of rotational 3D angiography in endovascular treatment of ruptured cerebral aneurysm. AJNR Am J Neuroradiol 2002;23:686–688 [PMC free article] [PubMed] [Google Scholar]
- 12.Kiyosue H, Okahara M, Tanoue S, Nakamura T, Nagatomi H, Mori H. Detection of the residual lumen of intracranial aneurysms immediately after coil embolization by three-dimensional digital subtraction angiographic virtual endoscopic imaging. Neurosurgery 2002;50:476–48511841714 [Google Scholar]
- 13.Okahara M, Kiyosue H, Yamashita M, et al. Diagnostic accuracy of magnetic resonance angiography for cerebral aneurysms in correlation with 3D-digital subtraction angiographic images: a study of 133 aneurysms. Stroke 2002;33:1803–1808 [DOI] [PubMed] [Google Scholar]
- 14.Sugahara T, Korogi Y, Nakashima K, Hamatake S, Honda S, Takahashi M. Comparison of 2D and 3D digital subtraction angiography in evaluation of intracranial aneurysms. AJNR Am J Neuroradiol 2002;23:1545–1552 [PMC free article] [PubMed] [Google Scholar]
- 15.Tanoue S, Kiyosue H, Kenai H, Nakamura T, Yamashita M, Mori H. Three-dimensional reconstructed images after rotational angiography in the evaluation of intracranial aneurysms: surgical correlation. Neurosurgery 2000;47:866–871 [DOI] [PubMed] [Google Scholar]
- 16.Yasargil MG. Microneurosurgery. Vol 1. Stuttgart: Georg Thieme Verlag;1984. :72–128
- 17.Teal JS, Rumaugh CL, Bergeron RT, Segall HD. Anomalies of the middle cerebral artery: accessory artery, duplication, and early bifurcation. AJR Am J Roentgenol 1973;118:567–575 [DOI] [PubMed] [Google Scholar]
- 18.Cornelis G, Bellet A, van Eygen B, Roisin P, Libon E. Rotational multiple sequence roentgenography of intracranial aneurysms. Acta Radiol Diagn (Stockh) 1972;12:74–76 [DOI] [PubMed] [Google Scholar]
- 19.Voigt K, Stoeter P, Peterson D. Rotational cerebral roentgenography, I: evaluation of the technical procedure and diagnostic application with model studies. Neuroradiology 1975;10:95–100 [PubMed] [Google Scholar]
- 20.Bosanac Z, Miller RL, Jain M. Rotational digital subtraction carotid angiography: technique and comparison with static digital subtraction angiography. Clin Radiol 1998;53:682–687 [DOI] [PubMed] [Google Scholar]
- 21.Elgersma OE, Buijs PC, Wust AF, van der Graaf Y, Eikelboom BC, Mali WP. Maximal internal carotid arterial stenosis: assessment with rotational angiography versus conventional intraarterial digital subtraction angiography. Radiology 1999;213:777–783 [DOI] [PubMed] [Google Scholar]
- 22.Pozzi Mucelli F, Pecenco R, Calderan L, Pozzi Mucelli R. Rotational angiography of the carotid artery bifurcation: technical aspects and preliminary results. Radiol Med (Torino) 2002;104:157–164 [PubMed] [Google Scholar]
- 23.Harrison MJ, Johnson BA, Gardner GM, Welling BG. Preliminary results on the management of unruptured intracranial aneurysms with magnetic resonance angiography and computed tomographic angiography. Neurosurgery 1997;40:947–957 [DOI] [PubMed] [Google Scholar]
- 24.Tu RK, Cohen WA, Maravilla KR, et al. Digital subtraction rotational angiography for aneurysms of the intracranial anterior circulation: injection method and optimization. AJNR Am J Neuroradiol 1996;17:1127–1136 [PMC free article] [PubMed] [Google Scholar]
- 25.Colombo F, Cavedon C, Francescon P, et al. Three-dimensional angiography for radiosurgical treatment planning for arteriovenous malformations. J Neurosurg 2003;98:536–543 [DOI] [PubMed] [Google Scholar]
- 26.Takahashi S, Hoshino F, Uemura K, Takahashi A, Sakamoto K. Accessory middle cerebral artery: is it a variant form of the recurrent artery of Heubner? AJNR Am J Neuroradiol 1989;10:563–568 [PMC free article] [PubMed] [Google Scholar]
- 27.Tran-Dinh H. The accessory middle cerebral artery: a variant of the recurrent artery of Heubner (A. centralis longa)? Acta Anat 1986;126:167–171 [PubMed] [Google Scholar]
- 28.Rosner SS, Rhoton AL Jr, Ono M, Barry M. Microsurgical anatomy of the anterior perforating arteries. J Neurosurg 1984;61:468–485 [DOI] [PubMed] [Google Scholar]