Abstract
BACKGROUND AND PURPOSE: The literature contains relatively few reports of distal embolism associated with intervention for intracranial atherosclerotic disease. Our purpose was to evaluate the frequency of thromboembolic events after percutaneous transluminal angioplasty (PTA) or stent placement in this setting by using diffusion-weighted (DW) imaging.
METHODS: Between October 1999 and January 2004, 16 consecutive patients with symptomatic intracranial arterial stenosis greater than 60% were treated with PTA or stent placement without a protection system. Whole-brain DW imaging was performed before and after intervention. DW imaging findings were retrospectively analyzed and divided into three groups according to new hyperintensities: type A was none; type B, a single lesion; and type C, multiple lesions.
RESULTS: Nine type A, five type B, and three type C lesions were detected after the interventions. All hyperintense lesions were less than 5 mm in diameter. All type C lesions occurred in the context of internal carotid artery stenosis treated with stent placement. DW imaging abnormalities occurred most frequently when PTA followed by stent placement was performed for long internal carotid artery stenoses. No new neurologic deficits occurred in any patient.
CONCLUSION: In this series, PTA or stent placement or both for intracranial atherosclerotic lesions was safe. New DW imaging abnormalities were less frequent in patients who underwent PTA alone or primary stent placement than in those receiving PTA followed by stent placement.
The rate of ischemic events in patients with symptomatic intracranial arterial stenosis is 7–9% per year (1, 2). Advancements in interventional neuroradiology have made the endovascular treatment of intracranial atherosclerotic disease possible (3–6). However, this procedure carries certain risks, including vessel rupture, occlusion of perforating arteries, and cerebral embolism. In carotid artery stent placement (CAS), major complications have been reported to occur with the distal embolism of plaque material during the procedure (7, 8). In contrast, the literature contains relatively few reports of distal embolism associated with intervention for intracranial atherosclerotic disease. Therefore, clinicians have performed intracranial percutaneous transluminal angioplasty (PTA) or stent placement without protection systems, as the rate of secondary thromboembolic complications was considered low.
Diffusion-weighted (DW) imaging is the most sensitive method to detect early ischemia. DW imaging has been useful in identifying silent ischemia after various endovascular treatments, such as diagnostic cerebral angiography, CAS, and aneurysmal coil placement (7–11). In this study, we evaluated the pattern and frequency of thromboembolic events after PTA or stent placement or both for intracranial atherosclerotic diseases by using DW imaging.
Methods
Patients
Between October 1999 and January 2004, 16 consecutive patients with symptomatic intracranial arterial stenosis greater than 60% were treated with PTA or stent placement or both. They included 14 men and two women, with an age range of 56–78 years (mean: 67.5 years). All patients had transient ischemic attacks or small cerebral infarctions that were believed to be secondary to stenosis of the intracranial artery. These diagnoses were evaluated by a neurologist (H.M.) who was blinded to the radiologic features. Ten patients had stenosis of the cavernous or petrous portion of the internal carotid artery (ICA), five patients had stenosis of the middle cerebral artery (MCA), and two had restenosis of the MCA. No cases of basilar or vertebral artery stenosis were included in this series. Because of restenosis, one patient underwent PTA twice. Overall, 17 procedures were performed in the 16 patients, and all underwent evaluation with DW imaging to assess embolic complications.
Endovascular Therapy
In this series, all endovascular procedures were performed under local anesthesia. MCA stenoses were treated with PTA alone, as the properties of the available stents made then unfavorable for navigation into the MCA lesion. In contrast, stenoses of the cavernous or petrous portion of the ICA were treated with stent placement; this approach was based on favorable patency outcomes in a previous report (3). Because no suitable device was available to prevent embolic complications, we did not utilize a protection device during these procedures.
In patients treated with PTA alone, the procedure was performed by using single-lumen (Fas Stealth; Boston Scientific, Boston, MA) or double-lumen (Maverick, Ranger, Gateway, Boston Scientific; SAVVY, Cordis Endovascular, Miami Lakes, FL; or Opensail, Guidant, Santa Clara, CA) PTA balloon catheters of 2–4 mm in diameter and 10–20 mm in length. A 0.014-inch guidewire was exchanged into the valve wire system in single-lumen PTA balloon catheters and used for navigation of the catheter. In double-lumen catheters, the tip of the guidewire was kept in the M2 segment to stabilize the balloon catheter. After correct positioning, the PTA balloon was slowly inflated and kept at 6–8 atm for 1–2 minutes. In seven patients treated with PTA alone, the number of balloon inflations ranged from one to four.
In patients treated with stent placement, we used one NIR stent (Boston Scientific), three Bx-Velocity stents (Johnson & Johnson, Miami, FL), and six S-670 stents (Medtronic, Minneapolis, MN). The diameter and length of the delivered stents varied from 3.0 to 4.5 mm and from 9 to 24 mm, respectively. In cases of high-grade stenosis, the stenotic lesion was slightly dilated with a PTA balloon to allow passage of the stent. If the stenosis was less than 70%, the stent was frequently delivered and deployed into the lesion without predilation. In all three patients receiving primary stent placement, postdilation was performed once at the proximal portion of the stent to be secured to the parent vessel. In one case invovling a petrous lesion within a tortuous ICA, a buddy-wire technique with another stiff guidewire (Stabilizer; Cordis Endovascular) was required for successful catheter navigation.
Overall, five MCA stenoses and two MCA restenoses were treated with PTA alone. Seven ICA lesions were treated with PTA followed by stent placement, and three ICA lesions were treated with primary stent placement.
Drug Regimen
For all patients, antiplatelet therapy (ticlopidine, 200 mg/day; Daiichi Pharmaceuticals, Tokyo, Japan) was started at least 1 week before intervention and continued for 3 months afterward. The activated clotting time was maintained at 250–300 seconds by means of systemic heparinization during the procedure and kept at this level for 1–5 days after the intervention (Table). No hemorrhagic complications occurred.
Characteristics of 16 patients treated for intracranial atherosclerotic disease
| Pt/Age (y)/Sex | Stenotic Portion | Stenotic Rate (%) |
Lesion Length (mm) | Antiplatelet Therapy | Endovascular Therapy | Device* | Anticoagulation (d) | DWI† |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Abnormality | Location | |||||||
| 1/58/M | L C4 stenosis | 70 | 0 | 9 | 31 d | PTA, stenting | Savvy 3.0 × 20, S670 4.0 × 12 | 3 | A | Not applicable |
| 2/58/M | R C4 stenosis | 85 | 0 | 20 | 10 d | PTA, stenting | Maverick 2.5 × 20, S670 3.0 × 24 | 1 | C | Frontal, parietal |
| 3/68/M | R C4 stenosis | 76 | 0 | 12 | 60 d | PTA, stenting | Gateway 3.0 × 15, Bx-Velocity 4.5 × 18 | 2 | C | Frontal, parietal, basal ganglia |
| 4/70/F | R C4 stenosis | 63 | 15 | 7 | 30 d | PTA, stenting | Open sail 2.5 × 10, S670 3.5 × 9 | 3 | B | Frontal (contralateral) |
| 5/71/M | R C5 stenosis | 67 | 0 | 13 | 19 d | PTA, stenting | Maverick 3.5 × 15, S670 3.5 × 15 | 1 | B | Frontal |
| 6/78/M | R C5 stenosis | 60 | 0 | 7 | 13 d | Primary stenting | S670 3.5 × 15 | 1 | B | Frontal (contralateral) |
| 7/59/M | R C5 stenosis | 70 | 0 | 10 | 27 d | PTA, stenting | Gateway 3.0 × 15, Bx-Velocity 3.5 × 13 | 1 | B | Frontal |
| 8/62/M | R C5 stenosis | 60 | 10 | 6 | 19 d | Primary stenting | NIR 4.0 × 9.0 | 5 | A | Not applicable |
| 9/74/M | R C5 stenosis | 68 | 0 | 15 | 26 d | PTA, stenting | Gateway 3.5 × 9.0, Bx-Velocity 4.5 × 18 | 1 | C | Frontal, basal ganglia |
| 10/76/M | L C4 stenosis | 60 | 0 | 9 | 9 y | Primary stenting | S670 4.0 × 12.0 | 1 | A | Not applicable |
| 11/76/M | ||||||||||
| Procedure 1 | R M1 stenosis | 70 | 40 | 7 | 7 d | PTA | Gateway 2.0 × 12, 2.5 × 12 | 1 | A | Not applicable |
| Procedure 2 | R M1 restenosis | 60 | 10 | 7 | 96 d | PTA | Gateway 2.5 × 12 | 1 | B | Parietal |
| 12/68/M | L M1 restenosis | 67 | 10 | 9 | 1 y | PTA | Ranger 1.5 × 20, 2.0 × 20 | 1 | A | Not applicable |
| 13/65/M | R M1 stenosis | 80 | 20 | 7 | 29 d | PTA | Fas stealth 2.0 × 10 | 5 | A | Not applicable |
| 14/64/F | R M1 stenosis | 70 | 30 | 6 | 41 d | PTA | Ranger 2.0 × 20, 2.5 × 20 | 1 | A | Not applicable |
| 15/56/M | L M1-2 stenosis | 80 | 20 | 4 | 9 d | PTA | Fas stealth 2.0 × 10 | 2 | A | Not applicable |
| 16/77/M | L M1 stenosis | 80 | 40 | 6 | 11 d | PTA | Gateway 2.0 × 12 | 1 | A | Not applicable |
Note.—No new deficits were observed.
Dimensions are in millimeters.
Type A is no hyperintensities; type B, a single lesion; and type C, multiple lesions.
MR Imaging
Whole-brain DW imaging was performed with an echo-planar sequence 1 day before and 1–3 days after intervention. An isotropic sequence was used (TR/TE/NEX, 4000/137/2; field of view, 240 mm; matrix, 96 × 128) with b values of 0 and 1000 s/mm2. Two neuroradiologists (O.M., H.Y.) who were blinded to the patients’ clinical status retrospectively reviewed the DW images. All new hyperintensities were interpreted as a sign of new embolic lesions after intervention. DW imaging findings were analyzed and divided in three groups according to the characteristics of new hyperintensities: type A was none; type B, a single lesion only; and type C, multiple lesions.
The relationship between DW imaging abnormalities and characteristics of the stenosis (e.g., length and stenotic rate), as well as differences in DW imaging abnormalities among those undergoing PTA alone, primary stent placement, and PTA followed by stent placement were compared by using the Mann-Whitney U test and the Fisher exact probability test. A P value of less than .05 was considered to indicate a statistically significant association. In these analyses, we eliminated the contralateral DW imaging-positive lesions, as recognized in cases 4 and 6, because these were considered to appear as a result of diagnostic angiography or manipulation of the guiding catheter. The stenotic rate was determined by dividing the stenotic diameter by the distal diameter of the ICA or MCA, as in the North American Symptomatic Carotid Endarterectomy Trial (12).
Results
Patient profiles and the corresponding procedures are summarized in the Table. Nine type A, five type B, and three type C lesions were detected after the interventions. All hyperintense lesions were less than 5 mm in diameter. All type B lesions were localized to the subcortex, and two of three type C lesions were localized to the subcortex and basal ganglia. Two of five type B lesions were present only in the hemisphere contralateral to the stenosis.
We noted three type C lesions, and all three consisted of lesions 12–20 mm in length. In contrast, stenoses shorter than 10 mm were associated with no lesions or with only single lesions. The length of the stenoses was significantly correlated with DW imaging abnormalities (P = .0067), but DW imaging abnormalities were not correlated with the stenotic rate (P = .9062).
Ipsilateral DW imaging abnormalities were detected in five of six patients treated with PTA followed by stent placement. In contrast, only single lesions were detected after nine procedures involving PTA alone or primary stent placement alone. Therefore, DW imaging abnormalities appeared more frequently after PTA followed by stent placement than after PTA alone or primary stent placement (P = .0110).
Despite these findings, none of the patients experienced new neurologic deficits related to the intervention.
Representative Cases
Case 2.—
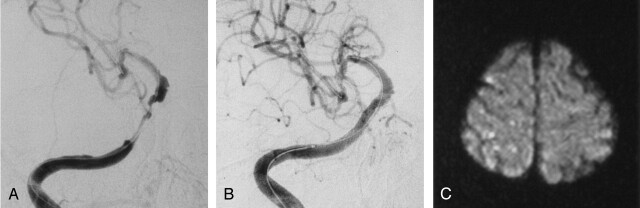
A 58-year-old man with dysarthria and left hemiparesis was admitted to our hospital. Angiography revealed an 85% stenosis of the right petrous ICA (Fig 1A). PTA was subsequently performed by using a 2.5 × 20-mm double-lumen balloon catheter (Maverick PTA balloon; Boston Scientific), and a 3.0 × 24-mm stent (S-670; Medtronic) was successfully placed across the lesion. The stenotic lesion was dilated completely, and the patient did not experience new neurologic sequelae (Fig 1B). DW imaging performed after the procedure showed type C lesions in the right hemisphere (Fig 1C).
Fig 1.
Case 2.
A and B, Right ICA angiograms. Pretreatment image in A shows long segmental stenosis of the right C4 portion. After stent placement, image in B shows good dilatation.
C, Postprocedural DW imaging shows type C lesions in the frontal and parietal subcortex.
Case 3.—
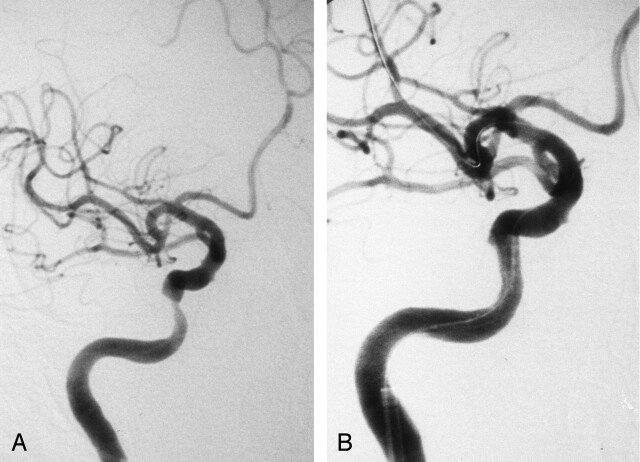
A 68-year-old man with dressing apraxia was admitted to our hospital. Carotid angiography showed 76% stenosis of the right petrous ICA (Fig 2A). After PTA with a 3.0 × 15- mm double-lumen balloon catheter (Gateway PTA balloon; Boston Scientific), we attempted to navigate a 4.5 × 18-mm stent (Bx-Velocity; Johnson & Johnson) through the tortuous ICA without success. Therefore, the petrous ICA was stretched with a stiff guidewire (Stabilizer; Cordis Endovascular) to allow for successful navigation of the stent. The stenotic lesion was then fully dilated, and the patient did not experience any new neurologic sequelae (Fig 2B). DW imaging after the procedure showed type C lesions in the right hemisphere.
Fig 2.
Case 3. Right ICA angiograms.
A, Pretreatment image shows elongated stenosis of the right C4 portion.
B, After stent placement, image shows good dilatation.
Discussion
Relatively few studies have been conducted to investigate the rate of distal embolism that occurs in the context of intervention for intracranial atherosclerotic disease. Indeed, we believe this is the first study to use DW imaging—a technique with high sensitivity for early detection of ischemic lesions (9)—to characterize the thromboembolic complication rate in the context of PTA or stent placement or both for intracranial atherosclerotic disease. DW imaging is a diagnostic technique with a high sensitivity for early detection of the ischemic lesions (9), and it can accurately depict insults within 40 minutes, and for at least 5 days after the onset of ischemic stroke (13–16).
In the present study, embolic complications occurred more frequently in the context of PTA followed by stent placement for ICA stenoses than in PTA-treated MCA stenoses or primary stent placement for ICA stenoses. Moreover, the length of stenoses was significantly correlated with DW imaging abnormalities. However, whether the difference in embolic complication rate is attributable to type of lesion, the type of treatment, or both is not clear because we did not compare PTA with stent placement in the same type of stenosis.
Ten ICA stenoses were treated with stent placement. All lesions were in the C4 or C5 portion of the ICA, and PTA or stent placement was performed without protection. Type C lesions were detected in three cases; all three consisted of stenoses longer than 12 mm. In fact, the relatively long length and tortuousity of the parent vessel made navigation of the stent difficult, and a stiff guidewire was required to stretch the tortuous ICA, as described in case 3. In cases such as this one, PTA or stent placement under proximal ICA occlusion and aspiration of debris, as Touho (17) reported, is considered effective in preventing embolic complications—but only when the ICA stenosis is proximal to the ophthalmic artery. Otherwise, embolism may not be prevented with proximal occlusion, because anastomotic flow is still present from the external carotid artery via the ophthalmic artery.
All hyperintense lesions in the present study were less than 5 mm in diameter. Sakai et al (8) classified hyperintense lesions after CAS and aneurysmal coil placement into five groups, and they reported on lesions larger than 5 mm in diameter after 12 of 154 procedures. Lovblad (7) also reported two cases of large hyperintense lesions in 19 patients undergoing CAS. On the basis of these results, it appears that large embolic lesions occur less frequently in PTA and stent placement for intracranial atherosclerotic disease than in CAS. Despite the fact that the intracranial arteries are essentially muscular arteries and the cervical carotid artery is an elastic artery, atherosclerosis can occur in either type of vessel. Ogata et al (18, 19) reported that plaque rupture or intraplaque hemorrhage can occur within a cerebral artery in a manner similar to that in the cervical carotid artery. However, the smaller diameter of the intracranial arteries corresponds to a smaller volume of atheromatous plaque. This may account for the decreased risk of distal embolism in the context of PTA or stent placement for intracranial atherosclerotic disease.
Conclusion
PTA or stent placement or both of intracranial atherosclerotic lesions was considered safe from our experience of 16 cases. New DW imaging abnormalities were seen less frequently in patients who underwent PTA alone or primary stent placement than in those who received PTA followed by stent placement. Particular care should be taken in long ICA stenoses, because it frequently caused DW imaging abnormalities.
References
- 1.Craig DR, Meguro K, Watridge C, et al. Intracranial internal carotid artery stenosis. Stroke 1982;13:825–828 [DOI] [PubMed] [Google Scholar]
- 2.Arenillas JF, Molina CA, Montaner J, et al. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke 2001;32:2898–2904 [DOI] [PubMed] [Google Scholar]
- 3.Terada T, Tsuura M, Matsumoto H, et al. Endovascular therapy for stenosis of the petrous or cavernous portion of the internal carotid artery: percutaneous transluminal angioplasty compared with stent placement. J Neurosurg 2003;98:491–497 [DOI] [PubMed] [Google Scholar]
- 4.Al-Mubarak N, Gomez CR, Vitek JJ, et al. Stenting of symptomatic stenosis of the intracranial internal carotid artery. AJNR Am J Neuroradiol 1998;19:1949–1951 [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez CR, Misra VK, Liu MW, et al. Elective stenting of symptomatic basilar artery stenosis. Stroke 2000;31:95–99 [DOI] [PubMed] [Google Scholar]
- 6.Yokote H, Terada T, Ryujin K, et al. Percutaneous transluminal angioplasty for arteriosclerotic lesions. Neuroradiology 1998;40:590–596 [DOI] [PubMed] [Google Scholar]
- 7.Lovblad KO, Pluschke W, Remonda L, et al. Diffusion-weighted MRI for monitoring neurovascular interventions. Neuroradiology 2000;42:134–138 [DOI] [PubMed] [Google Scholar]
- 8.Sakai H, Sakai N, Higashi T, et al. Embolic complications associated with neurovascular intervention: prospective evaluation by use of diffusion weighted MR image. No Shinkei Geka 2002;30:43–49 [PubMed] [Google Scholar]
- 9.Kato K, Tomura N, Takahashi S, Sakuma I, Watarai J. Ischemic lesions related to cerebral angiography: evaluation by diffusion weighted MR imaging. Neuroradiology 2003;45:39–43 [DOI] [PubMed] [Google Scholar]
- 10.Soeda A, Sakai N, Sakai H, et al. Thromboembolic events associated with Guglielmi detachable coil embolization of asymptomatic cerebral aneurysms: evaluation of 66 consecutive cases with use of diffusion-weighted MR imaging. AJNR Am J Neuroradiol 2003;24:127–132 [PMC free article] [PubMed] [Google Scholar]
- 11.Soeda A, Sakai N, Murao H, et al. Thromboembolic events associated with Guglielmi detachable coil embolization with use of diffusion-weighted MR imaging, II: detection of the microemboli proximal to cerebral aneurysm. AJNR Am J Neuroradiol 2003;24:2035–2038 [PMC free article] [PubMed] [Google Scholar]
- 12.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–453 [DOI] [PubMed] [Google Scholar]
- 13.Moseley ME, Kucharczyk J, Mintorovitch J, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol 1990;11:423–429 [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada N, Imakita S, Sakuma T. Value of diffusion-weighted imaging and apparent diffusion coefficient in recent cerebral infarctions: a correlative study with contrast-enhanced T1-weighted imaging. AJNR Am J Neuroradiol 1990;20:193–198 [PMC free article] [PubMed] [Google Scholar]
- 15.Lutsep HL, Albers GW, DeCrespigny A, et al. Clinical utility of diffusion-weighted magnetic resonance imaging in assessment of ischemic stroke. Ann Neurol 1997;41:574–580 [DOI] [PubMed] [Google Scholar]
- 16.Schlaug G, Siewert B, Benfield A, Edelman RR, Warach S. Time course of the apparent diffusion coefficient (ADC) abnormality in human stroke. Neurology 1997;49:113–119 [DOI] [PubMed] [Google Scholar]
- 17.Touho H. Percutaneous transluminal angioplasty in the treatment of atherosclerotic disease of the anterior cerebral circulation and hemodynamic evaluation. J Neurosurg 1995;82:953–960 [DOI] [PubMed] [Google Scholar]
- 18.Ogata J, Masuda J, Yutani C, Yamaguchi T. Rupture of atheromatous plaque as a cause of thrombotic occlusion of stenotic internal carotid artery. Stroke 1990;21:1740–1745 [DOI] [PubMed] [Google Scholar]
- 19.Ogata J, Masuda J, Yutani C, Yamaguchi T. Mechanisms of cerebral artery thrombosis: a histopathological analysis on eight necropsy cases. J Neurol Neurosurg Psychiatry 1994;57:17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]